Issue:May 2021
DRY-POWDER THERAPEUTICS - Respiration Inspiration: Local Treatment of Lung Cancer by Dry-Powder Inhaler
CHALLENGES IN LUNG CANCER TREATMENT
As of 2017, lung cancer was the leading cause of cancer-related deaths in Americans, with non-small-cell lung cancer (NSCLC) making up the majority of cases.1 Despite dozens of approved treatments for lung cancer, survival rates for advanced cases remain poor. One study found only one-third of Stage IV patients in a particular cohort remained alive and progression-free after 12 months of treatment.2 Overall, fewer than 5% of Stage IV patients survive for 5 years, despite intensive treatment with chemotherapy.3 Immunotherapy combination treatments can increase progression-free survival by up to 4 months.4
Effective lung cancer treatment faces two challenges. The first is the toxicity and/or side effects associated with systemic administration. Most lung cancer treatments are administered systemically, whether by injection, intravenous infusion, or the oral route. For example, chemotherapy-based treatments and large-molecule biologics for immunotherapy are administered by intravenous infusions and can be poorly tolerated by patients. The blood drug levels required for adequate lung tissue exposure are high enough that adverse effects are common.3,5 Many approved anticancer compounds are highly potent, but are rarely used due to severe toxicity. A second challenge to effective lung cancer treatment is the method of administration. Treatment by intravenous infusion must occur in a clinical setting, leading to high costs and challenges in patient compliance. For lung cancer, local delivery may provide a solution.
Site-specific (ie, local) treatment of lung cancer using an inhaled formulation offers an ideal means to overcome both of these challenges. The disadvantages of systemic administration can be overcome through local delivery using such well-established devices as dry-powder inhalers (DPIs), metered-dose inhalers, or nebulizers. There is a long history of treating lung ailments by such devices. DPIs are often preferred, due to their ease of use and product stability at ambient conditions. Issues with treatment convenience and compliance are also overcome with these devices, as patients can self-administer therapeutics at home. This is especially advantageous because lung cancer therapeutics, particularly immunotherapies, are often administered indefinitely as maintenance treatments.
The following describes the formulation and manufacturing considerations for development of dry-powder therapeutics for local treatment of lung cancer. Two case studies are presented in which two approved drugs — 5-azacytidine (5AZA) and topotecan — are successfully formulated for DPI administration to the lung.
FORMULATION & MANUFACTURING CONSIDERATIONS
To deliver any drug by inhalation, particle size is critical. The aerodynamic diameter of an aerosol particle, whether a solid for delivery by a DPI or liquid for delivery by a nebulizer, determines where the particles end up in the respiratory tract. For spherical particles, the aerodynamic diameter is a simple function of its geometric diameter and density (see equation 1 below):
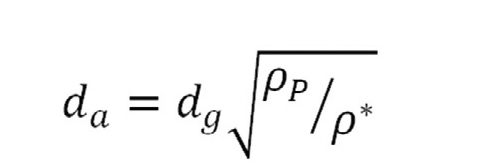
where da is the aerodynamic diameter, dg is the geometric diameter, ρP is the density of the particle, and ρ* is the reference density of 1000 kg/m3.6
Particles that are too large (those with aerodynamic diameters larger than about 5 microns) tend to get stuck at the back of the throat, failing to follow inhaled air around the 90-degree bend in the throat from mouth to trachea. Particles that are too small (below about 1 micron) fail to deposit in the lung and are exhaled out of the body. For optimum deposition of drug in the deep lung (that is, bronchioles and alveoli), aerosol particles with aerodynamic diameters of 1 to 5 microns are desired.6
In liquid-based delivery systems (nebulizer formulations), droplet size is controlled by an atomizer and compressed air. For solid-based delivery systems (formulations for DPIs), particle engineering is needed to achieve the target size distribution for pulmonary delivery. Micronization, often performed using a jet mill, can reduce the particle size of the active pharmaceutical ingredient (API) to the correct range. The API particles are then blended with a carrier, such as lactose, to prevent reaggregation of the micronized material.
Spray drying offers another flexible approach to create dry-powder particles with the correct size for inhalation. In this process, API and excipients are co-dissolved in a solvent and atomized into droplets, which are introduced into a drying chamber. Contact with hot drying gas rapidly removes solvent from the droplets, creating solid particles. The spray-dried dispersion (SDD) particles are separated from the outlet gas stream using a cyclone collector. By carefully tuning the droplet atomization and drying process parameters, an inhalation-ready powder can be manufactured. Some common spray-drying inhalation excipients include stabilizing sugars, such as trehalose, mannitol, and lactose, as well as the amino acid L-leucine. L-leucine is used in inhalation formulations to form a crystalline shell on the outside of particles, acting as a dispersant to improve aerosol performance.6 Further, we describe the successful use of spray-drying to prepare formulations for inhaled delivery.
CASE STUDY 1: 5-AZACYTIDINE
The first case study describes the preparation of a dry-powder 5AZA SDD for local inhaled delivery to the lung. The pharmacokinetic performance and efficacy of the SDD is compared to those of an inhaled aqueous formulation and an injectable systemic formulation.
5AZA is a chemotherapeutic that helps reduce the growth of cancer cells by inhibiting DNA methylation, a critical process in cell replication. The drug is currently approved for treatment via injection for myelodysplastic syndromes and leukemia and is in clinical trials for treatment of other cancers. When administered systemically, dose-limiting toxicity is often observed, which limits the compound’s effectiveness. 5AZA is an excellent candidate for local delivery to the lung because pulmonary delivery will reduce toxicity and minimize the effects of 5AZA on the DNA of healthy tissue.
Formulation & In Vitro Characterization
Spray drying was used to produce dry-powder SDD particles that contained 10% 5AZA, 70% trehalose, and 20% L-leucine by weight. X-ray diffraction showed that the L-leucine was crystalline, and thermal analysis showed a single amorphous trehalose/5AZA phase. The mass median aerodynamic diameter (MMAD) of the 5AZA SDD was measured with a Next Generation ImpactorTM (NGI) and a Plastiape low-resistance DPI device. The measured value of 3.6 +/– 1.6 microns was within the target range for delivery to the deep lung.
Manufacturing Approach
The greatest challenge in developing a 5AZA SDD was the poor chemical stability of 5AZA in water. 5AZA is easily dissolved in dimethyl sulfoxide (DMSO), but trehalose and L-leucine are not adequately soluble in DMSO for a single-solvent approach to be feasible. To combat this issue, a “solvent-shift” spray-drying process was used, in which an aqueous solution of trehalose and L-leucine was prepared and then combined with the 5AZA-DMSO solution using an in-line mixer just before atomization in the spray-drying process. This process limited exposure of the 5AZA to water to just a few seconds, preventing 5AZA degradation during manufacturing. This approach produced SDD powder with good aerosol properties, as well as adequate chemical and physical stability.
Pharmacokinetic Study
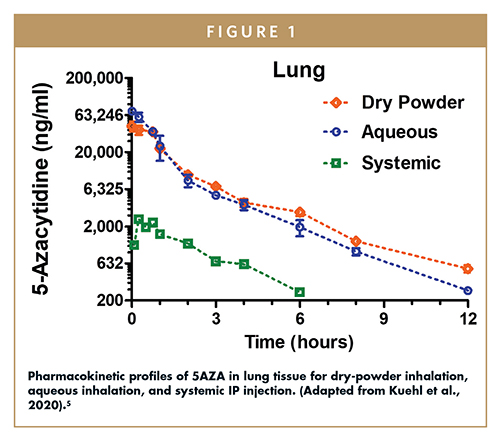
A pharmacokinetic study was conducted in rats to evaluate the levels of 5AZA in lung tissue for three administration routes: (1) systemic by intraperitoneal (IP) injection, (2) inhaled aqueous formulation, and (3) inhaled dry-powder SDD (Figure 1).5 The dry powder was aerosolized using a rotating brush generator and presented to the rats for inhalation through the nose. Both inhaled formulations increased the area under the curve (AUC) in lung tissue 14- to 15-fold compared to the AUC of the systemic formulation that was injected. When normalized for the reduced dose of the inhalation treatments, the AUC was 50-fold higher than that of the systemic formulation. This study demonstrated that local administration to the lung is an effective way to increase 5AZA levels in affected tissue, while avoiding large increases in systemic exposure.
Efficacy Study
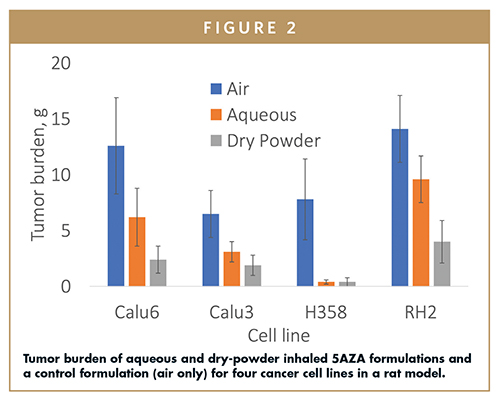
An efficacy study was conducted in an orthotopic nude rat model for NSCLC to assess the impact of 5AZA on tumor burden.5 Briefly, NSCLC cells were instilled intratracheally into the lungs of rats. Twelve cohorts were tested: control (air only), aqueous inhaled 5AZA, and dry-powder inhaled 5AZA were each administered to rats with one of four tumor cell lines. In all four cell lines, the two inhaled formulations reduced tumor burden significantly more than the control (Figure 2). The dry-powder formulation was found to be most effective in three cell lines, and equally effective in the fourth.
To confirm the mechanism of tumor burden reduction, the number of genes demethylated during treatment was quantified using RNA-sequencing analysis. The dry-powder formulation was found to be superior at transcriptional reprogramming of the genome, compared with the aqueous formulation. The dry-powder formulation delivered high levels of 5AZA to the lung, demethylating the DNA in diseased tissue and thereby reducing tumor burden in the rat model.
CASE STUDY 2: TOPOTECAN
The second case study describes the preparation of a dry-powder topotecan SDD for local inhaled delivery to the lung. Again, the pharmacokinetic performance and efficacy of the SDD was compared to that of an inhaled aqueous formulation and an injected systemic formulation.
The chemotherapeutic agent topotecan is a topoisomerase-I inhibitor used to treat a range of cancers. It was approved for use as an intravenous (IV) infusion in 1996 and as an oral formulation in 2007. Although it is effective at treating lung cancer, its use is complicated by severe hematological toxicity, which can limit dosing in some patients. Topotecan is an excellent candidate for local delivery to the lung because pulmonary delivery will maximize exposure of lung tissues and minimize systemic exposure to reduce toxicity.
Formulation & In Vitro Characterization
Spray drying was used to produce dry-powder topotecan SDD particles that contained 10% topotecan, 70% trehalose, and 20% L-leucine by weight. As topotecan has good solubility in a pH 3.5 aqueous solution, the SDD was manufactured by a standard, single-solvent process. The aerosol properties of the SDD were measured by NGI, showing an MMAD of 2.9 +/– 1.9 microns and a fine particle fraction (FPF) of 62% (% of emitted dose with aerodynamic diameter less than 5 microns). These results indicated a highly respirable dry powder with suitable characteristics for delivery in the deep lung.
Pharmacokinetic Study
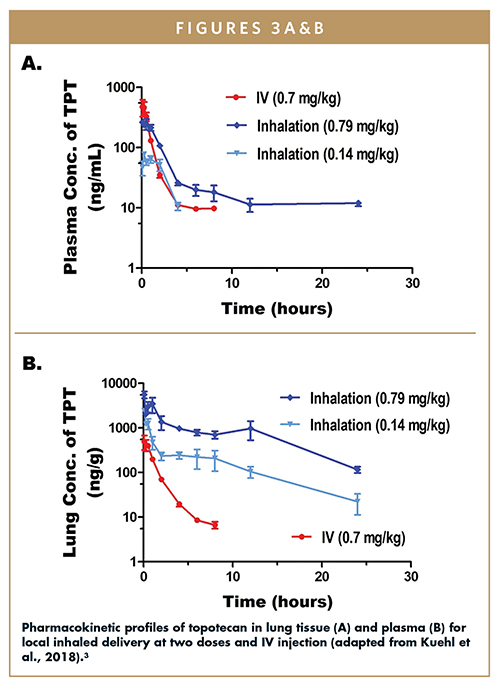
A pharmacokinetic study compared lung tissue and plasma levels for two doses of the inhaled dry-powder topotecan SDD formulation (0.79 and 0.4 mg/kg) and systemic (injected) topotecan (at 0.7 mg/kg).3 Pharmacokinetic curves for lung tissue and plasma are shown in Figure 3. In lung tissue (Figure 3A), the levels of topotecan were much higher for the two inhaled doses than for the injected systemic formulation. The dose-normalized AUC levels in the lung were 895 hr*kg*ng/mL*mg for the injected systemic formulation, and 34,092 and 27,647 hr*kg*ng/mL*mg for the low-dose the high-dose inhaled dry-powder formulation, respectively. Despite the high exposure in the lung tissue, plasma levels remained fairly low for both doses of inhaled topotecan (Figure 3B). The study confirms that a dry powder formulation can be used to deliver high levels of topotecan to the lung while maintaining low systemic exposure.
Efficacy Study
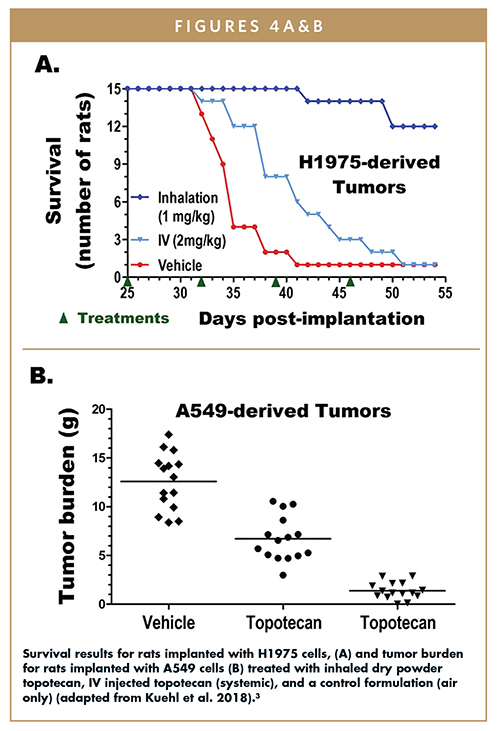
An orthotopic nude rat study was designed to compare the efficacy of inhaled and injected topotecan at reducing the burden of tumors grown from two human adenocarcinoma cell lines: (a) H1975, an aggressive and fast-growing cell line, and (b) A549, a moderate-growth cell line.3 After 25 days of tumor growth, rats were dosed weekly with either 1 mg/kg or 2 mg/kg of an inhaled dry-powder topotecan formulation, 2 mg/kg injected topotecan (systemic), or air only (as a control). Two results from the study are highlighted here. In the H1975 cohort, an aggressive and fast-growing cell line, rats who received the inhaled dry-powder formulation survived substantially longer than those who received the injected systemic or control treatment (Figure 4A). In the moderately-growing A549 tumor cohort, significantly improved tumor burden was observed for the rats who received the inhaled dry-powder formulation (Figure 4B). Taken together, these results demonstrate the promising impact of local administration of topotecan for treatment of lung cancer.
OUTLOOK FOR LOCAL TREATMENT OF LUNG CANCER
These case studies demonstrate the potential of local lung cancer treatment to improve efficacy, reduce dose, lessen risk of systemic side effects, and improve patient compliance by providing a convenient dosage form. Specifically, the pharmacokinetic studies demonstrate how greatly improved exposure to lung tissue can be achieved by the inhalation route. Efficacy studies show the impact of this improved exposure on tumor reduction with human lung cancer cell lines. The formulations of 5AZA and topotecan could lead to new options for treating local and metastatic lung cancer, for adjuvant therapy and, when combined with immunotherapy, improve patient survival.
From a manufacturing perspective, spray drying provides a flexible, scalable platform for developing inhaled lung cancer treatments of all varieties. From chemotherapeutics to kinase inhibitors to proteins, spray drying can be used to manufacture stable, respirable, and efficacious formulations for local pulmonary delivery. Multiple inhaled SDDs are currently in clinical trials for a wide range of lung indications, including pulmonary arterial hypertension, idiopathic pulmonary fibrosis, lung infection, and lung cancer. A locally administered treatment holds great promise for improved patient outcomes during primary and maintenance phases of lung cancer treatment. Contract development & manufacturing organizations (CDMOs) with appropriate SDD manufacturing capabilities may help advance these innovative cancer treatments to patients.
REFERENCES
- Russo, A.E., et al., Bevacizumab in the treatment of NSCLC: patient selection and perspectives. Lung Cancer (Auckland, N.Z.), 2017. 8: p. 259-269.
- Carbone, D.P., et al., First-Line Nivolumab in Stage IV or Recurrent Non–Small-Cell Lung Cancer. 2017. 376(25): p. 2415-2426.
- Kuehl, P.J., et al., Inhalation delivery of topotecan is superior to intravenous exposure for suppressing lung cancer in a preclinical model. Drug Delivery, 2018. 25(1): p. 1127-1136.
- Pirker, R., Immunotherapy combinations in advanced nonsmall cell lung cancer. Current Opinion in Oncology, 2021. 33(1).
- Kuehl, P.J., et al., 5-Azacytidine inhaled dry powder formulation profoundly improves pharmacokinetics and efficacy for lung cancer therapy through genome reprogramming. British Journal of Cancer, 2020. 122(8): p. 1194-1204.
- Vehring, R., Pharmaceutical Particle Engineering via Spray Drying. Pharmaceutical Research, 2008. 25(5): p. 999-1022.

Dr. Philip Kuehl is a Senior Scientist and Director of Scientific Core Laboratories at Lovelace Biomedical in Albuquerque, NM. He earned his Bachelor’s degree in Biochemistry from Hamline University in St. Paul, MN, and his PhD in Pharmaceutical Sciences from The University of Arizona, College of Pharmacy in Tucson Arizona. He is an Adjunct Professor at the University of New Mexico College of Pharmacy.

Dr. Kim Shepard is an Associate Principal Engineer in Research and Development at Lonza in Bend, OR. She earned her Bachelor’s and Master’s degrees in Chemical Engineering from Stevens Institute of Technology in Hoboken, NJ, and her PhD in Chemical Engineering from Princeton University. Her research interests include pharmaceutical spray drying, amorphous materials, and pulmonary delivery of therapeutics.
Total Page Views: 4793












