
Windgap Medical, Inc
200 Dexter Ave, Suite 270
Watertown, MA 02472
T: (617) 440-3311
E: bd@windgapmedical.com
W: https://windgapmedical.com/
LinkedIn: https://www.linkedin.com/company/windgap-medical
Windgap Medical offers drug delivery devices that simplify, automate, and accelerate the administration of complex injectables. With our patient-centric design approach, our products are designed to manage the challenges of reconstitution, liquid/liquid mixing, and sequential delivery of multiple liquid drugs. By reducing the number of user steps, our products fit easily into a patients’ daily life, enhancing usability and adherence. You focus on the formulation. We’ll deliver the solution.
Windgap’s injection devices are ideal for:
In partnership with pharmaceutical companies, we leverage our enhanced product features to improve outcomes for your life-changing molecules.
CORE CAPABILITIES
Mixing: Our automated approach to fluid management enables active, in device-controlled, mixing that eliminates shaking and swirling from the instructions for use. We provide a reliable endpoint that ensures a lyophilized (or powdered) drug is mixed the same way, every single time.
Multi-Liquid Delivery: In two simple steps, we can administer two liquid drugs through the same delivery needle. The perfect platform for drugs that can’t be co-formulated in the same container.
PRODUCT PLATFORMS
LVDC (Large Volume Dual Chamber) Platform: The novelty of Windgap’s LVDC platform stems from its innovative arrangement of standard off-the-shelf primary drug containers (PDC). The PDC architecture features side-by-side nesting of two, readily available, single-chamber cartridges with Windgap’s proprietary mixing and delivery needle hub. The side-by-side nesting permits use of ISO-compliant cartridges compatible with industry-standard filling methods, while maintaining a compact, easy-to-handle form factor.
The cartridges can be either plastic or glass; cartridge sizes from 1mL to 5mL. The design is compatible with standard aseptic filling methods or terminal sterilization strategies; and IM or SC needle lengths.
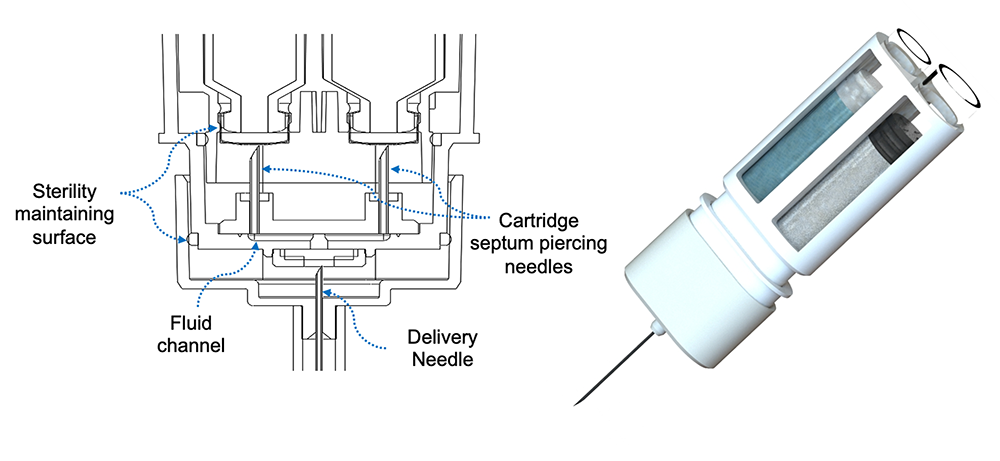
ANDI (Automatic recoNstitution Dual Chamber Injector) Platform: This offers a revolutionary twist on emergency autoinjectors. This compact device provides a thermally stable SC/IM drug delivery platform for automatic mixing and rapid dissolution of delivered doses up to 0.3 mL. ANDI is designed to meet the 99.999% reliability metrics for rescue applications. Windgap’s first combination product, the ANDIpen®, is being commercialized in partnership with ALK-Abelló to deliver emergency IM epinephrine doses.
INJECT SIMPLICITYTM INTO YOUR NEXT COMBINATION PRODUCT
Reach out via email or LinkedIn to discuss your molecule’s unique needs and opportunities to collaborate via partnership or feasibility studies.
Posted Date: 11/15/2023
This record has been viewed 5359 times.