Issue:June 2016
DRUG DEVELOPMENT EXECUTIVE - Crown Bioscience: Enhancing the Drug Development Process
 Crown Bioscience, Inc. is a preclinical CRO with expertise in the disease areas of oncology and metabolic disease. The company is known for the breadth and quality of its in vitro and in vivo models, as well as its ability to help clients quantify the real efficacy and pharmacological profile of their candidate before they move into the clinic. As drug discovery continues to rapidly evolve, there is a growing need to screen candidates earlier and with more patient-relevant models, which more closely reflect the situation in the clinic and therefore help improve the selection of drug candidates. Crown’s unique collection of ready-to-run, well-validated in vitro and in vivo models, expertise in model development, comprehensive drug discovery platforms, and global capacity enable them to deliver the results their clients need. Drug Development & Delivery recently interviewed Jean Pierre Wery, President of Crown Bioscience, to discuss the requirement for more accurate research models in oncology research, focusing on patient-derived xenograft (PDX) models that have the ability to more adequately represent the conditions and mechanisms of immunotherapy in human patients.
Crown Bioscience, Inc. is a preclinical CRO with expertise in the disease areas of oncology and metabolic disease. The company is known for the breadth and quality of its in vitro and in vivo models, as well as its ability to help clients quantify the real efficacy and pharmacological profile of their candidate before they move into the clinic. As drug discovery continues to rapidly evolve, there is a growing need to screen candidates earlier and with more patient-relevant models, which more closely reflect the situation in the clinic and therefore help improve the selection of drug candidates. Crown’s unique collection of ready-to-run, well-validated in vitro and in vivo models, expertise in model development, comprehensive drug discovery platforms, and global capacity enable them to deliver the results their clients need. Drug Development & Delivery recently interviewed Jean Pierre Wery, President of Crown Bioscience, to discuss the requirement for more accurate research models in oncology research, focusing on patient-derived xenograft (PDX) models that have the ability to more adequately represent the conditions and mechanisms of immunotherapy in human patients.
Q: For our readers who may not yet be familiar with your company, can you briefly discuss Crown Bioscience and what it offers?
A: Since 2006, Crown Bioscience has been building and developing a portfolio of unique resources to help our clients in their endeavors to bring improvements in patient outcomes. Our mission is to address the unmet needs of life sciences by creating and supplying innovative translational technologies and platforms. Our products and services have been profoundly expediting research for in vitro, in vivo, translational oncology, target validation, preclinical proof-of-concept, assistive clinical strategy design, and biomarker discovery.
Our comprehensive drug discovery services and unique portfolio of models for both oncology and metabolic diseases enable our customers to significantly accelerate and improve the quality of their decision-making process about which candidates to move into the clinic. As a preclinical CRO, we provide a unique range of models and services to help our clients accelerate their research and drug development efforts.
Crown’s fully integrated drug discovery capabilities increase the speed and efficiency of progressing hits to preclinical candidates. Our disease expertise and innovative translational research platforms enable our clients to successfully identify their most robust molecules and determine strategic therapeutic areas and indications to enhance success in Phase II & III clinical trials.
Q: What products and services does Crown Bioscience specialize in?
A: Crown Bioscience has an array of products, including antibodies, cell lines, and tumor samples, and we specialize in preclinical mouse models. We have developed the world’s largest commercial collection of Patient Derived Xenograft (PDX) models (HuPrime®), assays, and annotated databases (HuBaseTM, XenoBaseTM, and MuBaseTM) that our clients can use to conduct exhaustive evaluation of compound efficacy. With our well-characterized PDX models, we can conduct preclinical Phase II-like trials – HuTrialTM – to discover and evaluate predictive biomarkers before a single patient has been dosed. The majority of HuPrime models are maintained in passage ready for client projects. In conjunction with Crown’s capacity to perform large-scale studies screening multiple models in one study, we can perform HuTrial in months, not years.
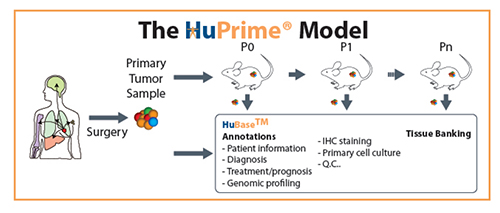
Our PDX models can be generated from a wide range of cancer types. They are usually implanted as fragments of tumors directly from patients to immunodeficient mice, and they generally retain the histological characteristics of the parental patient tumors. PDX models allow an invaluable assessment of tumor evolution and adaptive response to therapy and have been applied to preclinical drug testing and biomarker identification in a number of cancers, including ovarian, pancreatic, breast, and lung. These models have been shown to be biologically stable and accurately reflect the patient’s tumor not only with regard to histopathology, but also in its gene expression, genetic mutations, and therapeutic response. As an integral step in the oncology drug discovery process, cell line derived xenograft (CDX) models provide key decision-making information to allow an agent to move forward. Crown Bioscience has established multiple in vivo assay systems to evaluate novel anti-cancer compounds. Each assay is designed to understand specific aspects of each drug property and its mechanism of action. By using our comprehensive capability, our customers can advance development programs in a timely and cost-effective manner.
There are a total of around 2,000 cell lines available for studies; many of those have publically available profiling data. Crown Bioscience has recently launched the Phase II of Xenobase, a database that combines the publically available profiling data of more than 1,000 cell lines, with our proprietary in vivo pharmacology data. This allows users to make an informed decision by searching for gene mutation, amplification, and expression, as well as tumor growth in vivo and response to Standard of Care (SOC) treatments.
Q: What are some of the biggest challenges facing cancer research and the transition of new drugs from preclinical research to clinical trials?
A: The ability to maintain and study immortalised cell lines in an in vivo environment has proved to be a valuable tool in cancer research for several decades. These allow key decision-making information and a biologically relevant platform to study disease progression, develop novel therapies to improve treatment options, and allow an agent to move forward from preclinical trials. However, cancer cell lines have usually adapted to grow in culture. The lack of tissue architecture and heterogeneous population of cell types often abolishes cell-cell interaction, secretion, and other functions that depend on tissue context, and, therefore, cannot create an environment well-aligned with that of the patient’s tumor. Cells in culture are prone to genotypic and phenotypic drifting. Thereby cell lines can lose tissue-specific functions and acquire a molecular phenotype quite different from cells in vivo.
Another challenge facing cancer drug development is the high failure rate in clinical trials and the associated costs. Attrition rates in oncology are significantly higher than in other therapeutic areas, with only 5% of promising anti-cancer agents being licensed after successful Phase III trials. This is reflective of the lack of predictive power of traditional preclinical modelling in which efficacy at preclinical setting fails to translate into clinical benefit. The majority of failures are linked to efficacy rather than toxicity and, combined with the continually rising costs of clinical trials, have resulted in an oncology drug development process, which is both highly inefficient and enormously costly for pharmaceutical companies. In order to improve the efficiency and cost effectiveness of developing new therapies, current translational tools need to be enhanced to ensure they are of optimum value for indicating clinical success. Effective preclinical precision profiling screening platforms need to be established, which can validate the profile of a prospective drug candidate before entering clinical trials.
The use of relevant models can bring these high attrition rates down and offer a unique opportunity to study drug mechanisms within a tumor microenvironment relevant to patients.
Q: How is Crown Bioscience working to change the cancer drug development and clinical trial processes?
A: The evolution of clinical trials toward study types that find the correct targeted agent for the correct patient groups is crucial as the oncology drug development process is currently highly inefficient and needs a rapid overhaul to reduce attrition rates.
The key to improve drug discovery programs is the use of genomically characterized PDX models that are truly reflective of the patient population. This results in a model far more closely aligned with a patient’s disease than an immortalized cell line, allowing the assessment of tumor evolution and response to therapy.
Recent years have seen a considerable increase in the popularity of PDX models as a platform for the screening of novel therapeutics for the treatment of cancer. Their use in Phase II-like studies or human surrogate trials has significantly changed the way compounds are evaluated prior to transitioning into the clinical setting. By establishing deep biological insights into the pharmacological mechanisms of a drug and identifying potential biomarkers important to clinical trial design, Crown Bioscience’s HuPrime models provide drug developers with a significantly higher level of confidence for decision-making in the drug discovery process.
Q: How are your models uniquely qualified and able to address these challenges?
A: Our service can help pharmaceutical companies prioritize lead compounds, narrow down possible disease indication, and identify biomarkers to stratify patient populations in clinical trials, lowering drug attrition rates, and reducing development cost. Preclinical Phase II-like trials utilize a large cohort of PDX models with each PDX subject reflecting the pathology of its original patient (behaving as a patient avatar), and the cohort of patient avatars representing the diversity of the human patient population. Human surrogate trials can help to screen lead drug candidates, discover or validate predictive biomarkers and genetic signatures, and to position or reposition agents through identification of responder populations.
Crown’s HuPrime models, without in vitro manipulation, mirror patients’ histopathological and genetic profiles, and have been shown to have potential predictive power in the response from the same patients from whom they were derived. A large cohort of HuPrime can thus represent a wide range of disease diversity of patient populations. HuPrime has been increasingly recognized to be one of the most predictive animal models for evaluating anti-cancer therapies.
Q: What do you see as the trends for preclinical research in the future?
A: There is a growing requirement for more relevant models in the preclinical screening of modern drug candidates. By using models that are far more relevant to the clinical situation, in conjunction with precision molecular profiling and data analysis, it is possible to optimise and accelerate therapeutic compounds into the clinic. There is a great unmet need for improved preclinical models, with functional immunity, to drive forward promising research in immuno-oncology and to enable the successful transition of drugs from the laboratory to the clinic.
Models of murine immunity, including syngeneic and genetically engineered mouse models (GEMMs), can be used to interrogate novel immune treatments through activating the mouse immune system, follow the complete process of cancer progression and assess where stimulating the immune system is most beneficial. Newly developing platforms comprising allografts of spontaneous murine tumors, studied in mice with complete immunocompetency (MuPrimeTM) combine the improved predictive power of GEMM models with an operational simplicity, consistency, and robust growth for pharmacology research.
The oncology research community today is seeking better ways to offer a wider cross section of patients an improved quality of life and potential cures. There is a strong need to have tools available which can significantly improve the qualification of candidates at a much earlier stage in the drug discovery process. Our HuPrime models, as well as GEMM, MuPrime, and syngeneic models, can contribute by different means to move the most promising compounds to the clinic and reduce drug attrition rates by selecting the best clinical strategy.
To view this issue and all back issues online, please visit www.drug-dev.com.
Total Page Views: 2639

















