Issue:March 2013
CONTRACT SERVICES - Maximizing Yield in Transdermal Manufacturing
Introduction
Maximizing yields is critical, not only to ensure maximum return on investment on existing products, but also to opening the possibilities of new markets. With improved efficiency, pharmaceutical companies can pursue markets that in the past have been a challenge from a cost-benefit standpoint. Additionally, by maximizing manufacturing yield, a transdermal manufacturer can help lower the final cost of a drug product, benefiting end users as well.
As outlined in this article, efficient transdermal manufacturing requires knowledge and experience in formulation, expertise in each component of the process, as well as the interactions between manufacturing operations. Pharmaceutical companies must thoroughly vet their potential manufacturing partners to assess their strengths and weaknesses and determine if the manufacturer’s priorities align with the need for maximum yields. By working with a manufacturing partner who can provide proven expertise in every step of the process, pharmaceutical companies can give their products the best opportunity for high yields and profits.
Expanding Possibilities for New Markets
Passive transdermal drug delivery (TDD) offers a number of advantages to the pharmaceutical industry. This method of delivery is patient- and caregiver-friendly due to its simple method of selfadministration. It is painless and noninvasive, and for patients with serious or chronic conditions, it offers an easy way to take one less pill each day. Transdermal delivery also offers several advantages in compliance, as sustained-release patches can deliver a drug for up to 7 days, and the patch’s presence provides visual confirmation for patients or caregivers that the drug is being delivered (as opposed to having to wonder or try to remember if a pill was swallowed).
In terms of efficacy, administration of a drug via transdermal delivery allows the opportunity to avoid the first-pass metabolism of the drug in the liver. An additional advantage of transdermal delivery is the opportunity to reduce the chance of side effects, which can come from gastrointestinal exposure. Use of a patch also maintains constant therapeutic drug levels. Finally, an additional safety benefit provided by transdermal delivery is that it enables quick removal of the drug source in the event it becomes necessary.
These advantages of transdermal delivery highlight some of the reasons it is well received by patients. Unfortunately, in today’s pharmaceutical market, transdermal delivery is still an underutilized tool. This can be attributed to a number of factors, including the inclination of pharmaceutical companies to think primarily in terms of oral delivery when developing a new drug. It may also be related to a lack of understanding of how transdermal products are developed. However, many pharmaceutical companies are finding that with the right manufacturing partner, creating a product for transdermal delivery can be a smooth and predictable process. To give themselves the best odds for success in partnering with a manufacturer, pharmaceutical companies should be aware of the specific steps of the transdermal manufacturing process, as seen in Figure 1, and should challenge manufacturers to optimize their efficiency and yield.
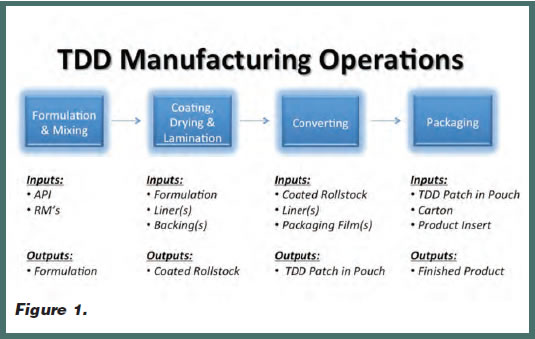
TDD Manufacturing Overview
The transdermal manufacturing process is composed of several manufacturing operations, specifically including formulation, mixing, coating, drying, lamination, converting, and packaging. The equipment used in these functions includes a combination of isolators, mixers, coaters, web lines, ovens, lamination, converting, and packaging equipment. While some may assume these operations represent the entire manufacturing process, it is important to understand there are also critical quality control parameters and measures at each step, as well as final product testing, to ensure all of the product’s requirements are met.
Optimizing the Process
In order to achieve the highest possible yields, manufacturers must first develop robust and predictable processes for each step of the operations. There are a number of tactics that can be used to achieve this. Broadly speaking, a manufacturer that leverages data-driven continuous improvement principles and methodologies such as Lean Six Sigma will be better prepared to achieve maximum productivity and yields. These methodologies go beyond the use of statistics and decision-making tools. It is really about the data-driven decision-making culture that is created in an organization that leverages these methodologies to make the on-going daily decisions that are critical to the quality of the product. Pharmaceutical companies should make inquiries to potential manufacturers about their formalized approach to continuous improvement.
There are a number of actions that manufacturers can and should take in relation to any given active pharmaceutical ingredient (API) and drug product to ensure high yields. These actions include optimizing procedures, training personnel, modifying existing equipment, and designing new equipment customized specifically for the requirements of the product and partner.
The overall goal in these endeavors is to ensure quality, improve productivity, reduce variation, and eliminate any non-value-added steps in the process, which consume valuable time and also increase the waste of raw materials. By engaging employees in open dialogue and hands-on training you can increase communication and understanding while harnessing their expertise to continuously apply best practices and optimize procedures. By designing and fabricating customized equipment for the specific process and product, manufacturers can help eliminate unnecessary steps, improving efficiency and yields.
As with any manufacturing process, it requires all process steps to hit yield targets to achieve the optimal rolled throughput yield for the product. However, this following will focus on formulation, mixing, coating, and converting operations to highlight how they can be leveraged to maximize yield.
For the formulation and mixing operation, a manufacturer must first understand the formulation requirements. This includes understanding if it is a solution or suspension, as well as other critical requirements, such as order of addition, percent solids, viscosity, potency, and batch size. This leads the manufacture to the selection of an appropriate mixing technology. To minimize waste and make sure employee health and safety are not compromised during formulation, manufacturers must examine how to properly mix, what kind of vessels to use, and how to minimize the amount of manual operation required. The manufacturer must consider any transfers or additions necessary when proceeding to the coating step. By considering not just the basic steps themselves but the intermediate steps between them, transdermal manufacturers can uncover additional opportunities for efficiency and improving yields. Selection of the optimal mixing technology directly impacts solvent and excipient use and concentrations, mix times, API yields, and ultimately wet mix homogeneity while protecting the safety and health of personnel. Optimizing the formulation and mixing process produces a stable, homogenous, and high-yield formulation that is fit for the coating operation.
Manufacturers often leverage commercially available coating methods, such as conventional roll-coating or die-coating methods. Oftentimes, these coating methods are part of the manufacturer’s base capabilities, but they may not be optimized for the specific formulation rheology or the specific product requirements, which can often directly impact yield loss.
Coating processes are critical to the overall product quality and yield of TDD products. A non-robust coating process can often consume costly processing time and expensive raw materials to set up the process and achieve the proper coat weight. Once in operation, a non-robust coating process can have unacceptable cross-web thickness uniformity or down-web thickness variation that does not meet product requirements. This can result in a manufacturing process that necessitates stopping, continuous adjustment, and rechecking throughout a batch. This directly impacts product yield via poor formulation yield. In addition, a nonrobust or poorly understood process may require extra in-process testing to ensure critical product requirements are being met. This causes further delays in the coating process and inefficient use of quality control laboratory personnel and equipment resources.
However, by performing thorough mathematical modeling in advance, a manufacturer can develop a customized coating method designed for and dedicated to a specific formulation. Figure 2 illustrates a direct comparison of a conventional and an optimized coating method using the same formulation. There are eight samples for each method comparing the coat weight versus target. The conventional coating method was adjusted to the desired coat width and thickness; however, despite these set-up and operational adjustments, it still displayed relatively high variation. The optimized coating method was mathematically modeled, and a die was designed and fabricated for the same specific formulation. It too was adjusted for coat width and thickness. However, in this case, the data demonstrates a significant improvement in the target coat weight compared to the acceptance limits. The optimized coating method delivers better coating uniformity, which directly impacts the manufacturing yield.
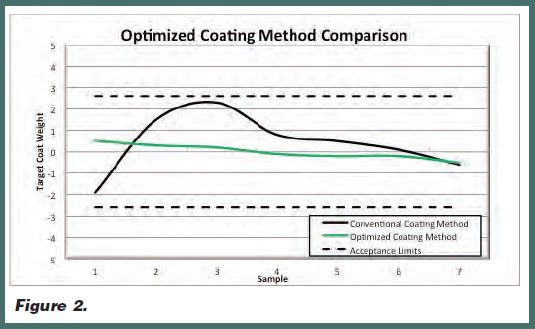
Specifically, by utilizing mathematical modeling, manufacturers can develop coating methods that reach the target coat weight more rapidly, enabling them to minimize formulation waste. Additionally, the steady coat weights throughout the coating run allow for excellent formulation and drug in adhesive (DIA) coated rollstock yields.
In the next operation, converting the process transforms the web-based DIA coated rollstock into a final converted patch system that is pouched and sealed in packaging film. As you can imagine, poor coating quality can result in downtime, splices, additional testing, and rejects at converting. This directly impacts converting run time, rates, and yields. Furthermore, the manufacturer should have flexible yet optimized converting equipment to maximize yield. A manufacturer should have experience with a variety of TDD product constructions, patch sizes, and wide-range of materials. This level of experience offers a valuable insight into their equipment design and process capabilities as well as their process knowhow. The manufacturer should have converting equipment that maximizes the utilization of coated DIA rollstock as well as liners and backings. Additionally, they should include inspection systems to continuously characterize the quality of the product being produced.
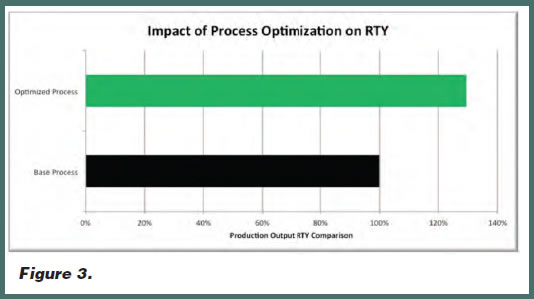
Figure 3 compares the rolled throughput yield (RTY) of a base TDD manufacturing process versus an optimized TDD manufacturing process. This rolled throughput yield improvement is ultimately realized in the converting process but is really a culmination of the improvements in the formulation, mixing, coating, and converting operations. For this specific product, it resulted in nearly a 30% rolled throughput yield improvement, which highlights the importance of process understanding.
These examples illustrate the importance of considering yields in each individual step of the manufacturing operation, but these steps are not the complete process. A manufacturer must also consider the interaction with the next step in the process, and how these interactions can be optimized as well.
Look for the Track Record
In seeking a manufacturing partner, pharmaceutical companies should look for one that has a history and expertise in each step of the manufacturing process. This includes assessing the vertical integration of the manufacturer. The manufacturer should also be able to optimize and customize its manufacturing processes for the company’s specific transdermal needs.
While it is true that any number of organizations can procure commercially available equipment and components needed for transdermal manufacturing, pharmaceutical companies should apply higher standards in order to find a manufacturing partner who can add maximum value by optimizing yields. An experienced manufacturer will be able to apply intellectual property and process knowhow in each step of the process. This kind of expertise is gained only from years of experience working with different types of formulations, whether they are solutions or suspensions, potent or non-potent compounds, and in various batch sizes ranging from small bench-top to large manufacturing campaigns. With this kind of history, a manufacturer can leverage its knowledge to optimize the process, determine best practices, and continuously apply new insights to the next program. A strong manufacturing partner provides not only a breadth of materials, but also a depth of knowledge that helps ensure success.
This depth of knowledge can be evidenced in a number of ways, including the use of statistical tools to perform tasks like modeling of coating methods based on specific rheology formulations, or modeling oven parameters to conduct designed experiments for optimization. Additionally, varied capabilities in lamination and web handling can allow a manufacturer to handle constructions from a monolithic adhesive layer to a multi-layer laminate construction. Intellectual property and process know-how help each step of the manufacturing process flow more smoothly.
It can be difficult to quantify the increase in yield that is possible with optimized procedures, and clearly this number will be different for every product. However, case studies exist that demonstrate double-digit improvements in yields when the proper protocols and equipment are put into place. These improvements are made evident in the converting and packaging steps, but are the cumulative result of improvements throughout the entire process – not just the final process step.
Maximizing Yields in Pain Management Products
While there are many areas of the pharmaceutical market that have yet to widely adopt transdermal delivery, the pain management market has embraced this method. Transdermal delivery is appealing for pain management drugs due to its consistent delivery of the API, and the successes and trends seen there are illustrative of what is possible for the industry overall.
Manufacturing pain management products for any delivery method can inherently have many challenges, as these products use DEA Schedule II or III potent compounds. Because of that, there are many barriers and engineering controls that must be put into place in order to manage the environmental, health, and safety risks to workers producing or conducting the manufacturing steps. Additionally, even if the APIs being used are not in limited supply, they are often high in cost. Therefore, maximizing the yield of the API is critical – not only in the manufacturing stage, but also during the development process.
A reliable manufacturing partner can take steps to effectively use these valuable materials to obtain the best possible data from the development process, and can also lay the groundwork to deliver the best payback when the manufacturing stage is reached. This can be predictably accomplished via the aforementioned tactics by minimizing the number of operations, eliminating non-value added tasks in the process, designing and fabricating custom equipment, implementing the proper procedures, engineering controls, and bringing the knowledge and expertise of working with various potent compounds. Not only do these practices reduce risks for workers at the manufacturing site, but also for the pharmaceutical company itself. Taking care in these steps delivers maximum yield for both the manufacturer’s and the pharmaceutical company’s needs.
While the challenges presented by manufacturing pain management products can be significant for some manufacturers, for the right manufacturer, pain management products can present an opportunity. Those that can successfully identify solutions to allow manufacturing processes to take place safely and effectively, while still providing all the benefits customers need, can better compete and articulate the value they bring to the process.

James Fenton
Plant Manager
3M Drug Delivery Systems
James Fenton is the Plant Manager for 3M Drug Delivery Systems at the division’s Process Technology Center (PTC), which is dedicated to the development and manufacture of transdermal and inhalation drug delivery products. Mr. Fenton has 15 years of experience at 3M in various technical and leadership roles within multiple divisions of the company, including process and product development. In addition, he has held a technical leadership position in a rapidly growing manufacturing operation and previously held technical leadership roles in 3M’s Corporate Research and Development Process Laboratory. Mr. Fenton earned his BSc in Chemical Engineering from the University of North Dakota.
Total Page Views: 4678

















