Issue:April 2014
FIXED-DOSE COMBINATIONS - Fixed-Dose Combination Products – A Review (Part 2 – Analysis)
INTRODUCTION
An analysis of fixed-dose combination (FDC) products can provide insight into evolving therapeutic needs and point the way to new product opportunities. In the first part of this series (Drug Development & Delivery, March 2014) we defined what we meant by FDC products and provided a general sense of the magnitude of these products. A review of that first article will provide a useful introduction to the analysis offered here. Note that this review is restricted to products approved in the US from 1990 through the end of 2013, and information was sourced using the PharmaCircle Product & Pipeline and FDA Products modules.
Of the 131 FDC products approved by the US FDA since 1990, it was previously noted that the majority of these products comprise pharmaceutical actives that had been previously approved. This is not surprising as the approval process for a new chemical entity (NCE) is sufficiently challenging that combining two or more unapproved actives substantially increases the associated risks and the costs. It is perhaps better to gain approval as a single entity NCE, properly understand its characteristics in a large population, and then pursue additional combination opportunities.
CORPORATE SPONSORS
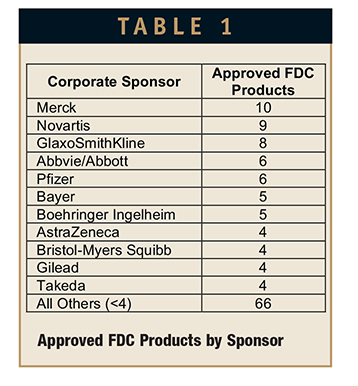
Of the 131 approved FDC products, a total of 87 (66%) were developed by, or in active partnership with, Big Pharma companies. Among the leading companies were Merck with 10, Novartis with 9, and GlaxoSmithKline with 8 FDC products. A list of companies is provided in Table 1. Specialty Pharma companies accounted for an additional 42 FDC products. The leading companies in this regard were Gilead with 4 and Valeant with 3. The remaining Specialty Pharma companies for the most part developed 1 or at most 2 combination products. Emerging biopharmaceutical companies, those without any product revenues, accounted for the remaining 2 approved FDC products.
THERAPEUTIC TARGETS
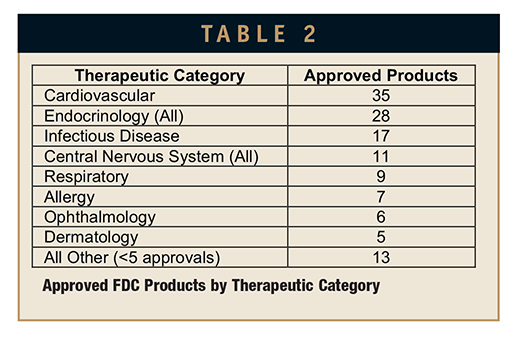
The most popular therapeutic indication for FDC products was cardiovascular with a total of 35 products (28 for hypertension). Endocrinology-related products followed with a total of 28 products. These endocrinology products covered a mixed bag of indications, including diabetes (13) and female health indications (12). The female health indications included contraception (9) and post-menopausal indications (3). It’s interesting to note how many “new” combinations of an estrogen and progestin have been introduced in the past 2 decades. This is a well-defined product opportunity path that is relatively low risk in terms of approval outcomes; clinical endpoints and trial designs have been well refined throughout the past few decades.
Infectious disease FDC products are also well represented with a total of 17 products. This may be a surprising number until one considers that the treatment of Human Immunodeficiency Virus (HIV) infections has been revolutionized by the combination of multiple antiviral agents, rather than single agents as is common with antibiotic treatments. Combining these agents in a single dosage form significantly improves outcomes by ensuring better compliance.
The only other double-digit therapeutic class was central nervous system with 11 FDC product approvals. The majority of these products were targeted to the management of pain, often combining 2 synergistic analgesics, or an analgesic and an antagonist.
The one other therapeutic area worth mentioning, because it represents an outsized share of FDC product sales, is respiratory disease. The past couple of decades have seen the development, approval, and wide market acceptance of inhaled combinations of a steroid and a bronchodilator for the management of asthma, and a steroid plus an anticholinergic for the treatment of chronic obstructive pulmonary disease. The leader in this area, GlaxoSmithKline’s Advair, a combination of fluticasone and salbutamol, has racked up more than $75 billion in worldwide sales since its first approval in 1999. The success of Advair has led other companies to introduce competing FDC products for the treatment of asthma and other respiratory indications, some of which have achieved sales in excess of $1 billion annually. A list of product approvals by therapeutic area is presented in Table 2.
DELIVERY ROUTES
It will be no surprise that the majority of approved FDC products are oral dosage forms (98/131). This is followed by topical FDC products (8). Inhalation, injectable, and ophthalmic delivery each accounted for 6 approved products.
BILLION-DOLLAR CLUB
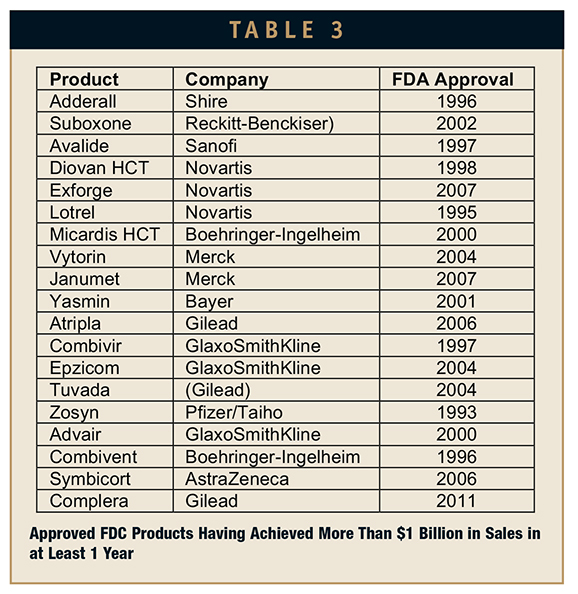
Of the 131 FDA-approved FDC products, a total of 19 (15%) have managed to capture more than a billion dollars in annual sales. GlaxoSmithKline and Novartis each have 3 products in the billion-dollar club, as does Gilead with its enviable portfolio of HIV combination products. Merck and Boehringer-Ingelheim follow with 2 products each.
Cardiovascular and infectious disease are the leading indications for these billion- dollar products. Infectious disease products include 5 HIV/AIDS and 1 antibiotic combination product. The cardiovascular products are a mixed bag of antihypertensive products combining actives with complementary activities, for example, a calcium channel blocker and an angiotensin receptor blocker. In two cases, a hypertensive and diuretic combination product was able to crack the billion-dollar annual sales mark solely on the basis of the combination product’s performance.
Although only 3 respiratory products managed to crack the billion-dollar annual sales mark, they did so with distinction. The many formulations of Advair (salbutamol/fluticasone) have achieved more than $8 billion of annual sales. Both Combivent and Pulmicort are members of the club and have proven to be very profitable products largely exempt from generic competition by virtue of their device-based delivery systems. Members of the Billion Dollar FDC Club are presented in Table 3.
GLOBAL VERSUS REGIONAL
It’s not surprising that the majority of the 131 FDA-approved FDC products have been approved in multiple markets around the world. What is surprising is that fully 41 of the total number of 131 products have only been approved in the US, or Canada and the US. Some of these US-only products are really not surprising and reflect the uniquely American attraction to opioid analgesics, often in combination formulations to deter abuse. But the US only status of Lotrel (amlodipine/benazepril), an antihypertensive, seems a bit odd. Looking a little more closely, there may be an opportunity for a company to take some of these US-only FDC combination products and see if there is an untapped international opportunity.
INNOVATION & CREATIVITY
When one looks over the list of FDC products approved since 1990, it’s hard to be impressed with the creativity of the developers. Many of the products seem to be slight variations on well-validated themes. Three common and decades-old themes stand out among the FDA product list. They are: the combination of a novel progestin with a validated estradiol analog (12 products), a novel antihypertensive with a diuretic, generally hydrochlorothiazide (20 products), and a novel antihistamine with pseudoephedrine (7 products). Together they account for 30% of all FDA-approved FDC products since 1990. The past couple of decades have seen four new themes developed and validated, with a concurrent introduction of multiple products built on the themes. These newer themes are a novel steroid combined in a single dosage form with a validated beta-agonist, or a novel beta-agonist with a validated steroid (4 products), a novel antidiabetic agent with metformin (9 products), the combination of multi-specific anti-HIV agents (8 products), and the combination of a narcotic agent with a non-narcotic analgesic, most often, it seems to avoid unfavorable prescription scheduling (5 products).
The concept of combining two or more agents in a single dosage presentation to ensure better compliance has had a major impact on the improved outcomes seen with HIV medications. The combined use of these agents is critical, but no less than ensuring compliance in taking all of the products in the prescription “cocktail.” The FDC combination of a narcotic with a narcotic antagonist must also be considered as novel and therapeutically important. In the case of Pfizer’s Embeda, the intention is to limit abuse while providing a therapeutically meaningful analgesic effect. The commercial success of this product, a US-only item, has been significantly impacted by the on-and-off-again issues related to formulation integrity of the dosage form. A far greater success has been seen with Reckitt-Benckiser’s Suboxone, a combination of buprenorphine and naloxone, targeted to the treatment of opioid addiction. This product is a member of the billion-dollar club.
Recognition should also be given to the increasing development of FDC products for the treatment of ophthalmic indications. Not only do these combined agents act in an additive or synergistic manner; they also help to ensure compliance by simplifying the dosing process. In the case of ophthalmic dosing, it’s not a simple matter of taking two pills rather than one.
FUTURE DEVELOPMENTS
The best way to understand what the future might bring is to look at the FDC products pipeline. That will be the focus of the third and final article in this series. We’ll take a look at the pipeline products from Phase I to those filed for approval in the US and the European Union to see what new trends and themes are working their way through to approval.
To view this issue and all back issues online, please visit www.drug-dev.com.

Dr. Tugrul T. Kararli earned his PhD in Pharmacology from the University of Florida and his MBA from DePaul University. Dr. Kararli worked at Searle/Pharmacia for 18 years and held various positions and responsibilities within the Pharmaceutical Sciences department, participating in pharmaceutics, product development, and drug delivery activities. As the Chairman of the Global Drug Delivery Technology Team at Pharmacia, he was responsible for identifying, planning, and executing the drug delivery technology strategies for marketed and development products. Dr. Kararli has authored numerous articles on various aspects of pharmaceutics and drug delivery and holds more than a dozen US and international patents. Currently, he is the Founder and President of PharmaCircle LLC, a knowledge management service company in the drug delivery and pharmaceutical/biotechnology fields.

Mr. Kurt Sedo is Vice President of Operations at PharmaCircle LLC. He earned his BS in Chemistry and Mathematics from the University of Wisconsin Stevens Point. Prior to joining PharmaCircle in 2003, he held various R&D Scientist positions within Searle/Pharmacia’s Pharmaceutical Sciences Department in Analytical Development and Drug Delivery.

Dr. Josef Bossart is Managing Director of The Pharmanumbers Group, a boutique research and consulting group providing the biopharmaceutical industry with analysis and insights that improve business outcomes. He has more than 3 decades of experience in the biopharmaceutical sector, including senior sales, marketing, business development, and management positions within Big Pharma, Specialty Pharma, and Emerging Pharma companies. He earned his PhD in Medicinal Chemistry from The Ohio State University, College of Pharmacy.
Total Page Views: 5961