Rani Therapeutics Announces Preclinical Pharmacodynamic Data on Transenteric Delivery of GLP-1 Incretin Triagonist
Rani Therapeutics Holdings, Inc. recently announced pharmacodynamic data of an incretin triagonist of the GLP-1, GIP, and glucagon receptors delivered transenterically, which mimics the RaniPill route of administration. This follows Rani’s prior study demonstrating oral delivery of a GLP-1 receptor agonist with high bioavailability via the RaniPill capsule.
“The preclinical data announced today are highly encouraging, as the data highlights that transenteric delivery mimicking the RaniPill’s route of drug administration results in pharmacodynamics comparable to subcutaneous injection for an incretin triagonist,” said Talat Imran, Chief Executive Officer of Rani Therapeutics. “In a previous preclinical study, Rani showed that the RaniPill capsule can orally deliver a GLP-1 receptor agonist with bioavailability and pharmacokinetics comparable to a subcutaneous injection. In the recent study of an incretin triagonist, Rani obtained pharmacodynamic data showing weight loss and reduction in serum lipids comparable to that observed via the subcutaneous injection route. We believe these data are reflective of the potential contributions our RaniPill capsule can make to the GLP-1 receptor agonist space and the broader obesity market. With the RaniPill platform, we have the potential to create oral alternatives for single and multiagonist drugs with differentiated dosing flexibility. We are evaluating our options with an intention to move forward with one or more products in this space.”
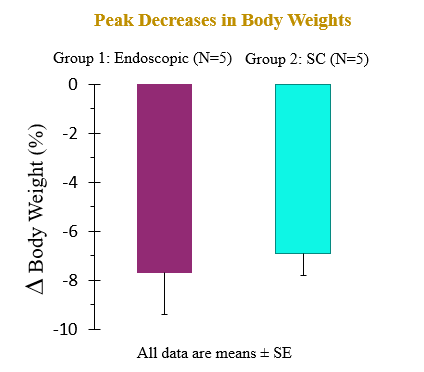
The recent preclinical study evaluated the pharmacokinetic (PK) and pharmacodynamic (PD) profiles of an incretin triagonist (GLP-1, GIP, glucagon receptors) when delivered via an endoscope-guided transenteric administration to mimic the RaniPill route of administration, versus the traditional administration route of subcutaneous (SC) injection. The study was conducted in canines separated into two groups. In Group 1 (N=5), 0.12 mg/kg of drug was administered via transenteric delivery by endoscope. In Group 2 (N=5), 0.12 mg/kg of drug was administered by subcutaneous injection. Blood samples were collected over 2 weeks for analysis of serum drug concentrations and various PD and safety biomarkers.
A single dose of drug delivered via either transenteric or SC routes elicited rapid decreases in body weight and serum lipids. Weight loss is believed to be due to early satiety leading to reduced caloric intake. The drug was well tolerated in both groups with no serious adverse events (SAEs) observed or changes in safety markers examined.
Near-Term Milestone Expectations
- Initiation of Phase 2 clinical trial of RT-102, a RaniPill GO containing teriparatide for osteoporosis, expected in 2024.
- Topline results of Phase 1 clinical trial of RT-111, a RaniPill GO containing ustekinumab biosimilar CT-P43, expected in the first quarter of 2024.
- Development of RaniPill HC to be ready for potential Phase 1 clinical trials in the second half of 2024.
Rani Therapeutics is a clinical-stage biotherapeutics company focused on advancing technologies to enable the development of orally administered biologics and drugs. Rani has developed the RaniPill capsule, which is a novel, proprietary and patented platform technology, intended to replace subcutaneous injection or intravenous infusion of biologics and drugs with oral dosing. Rani is progressing two RaniPill capsules, the RaniPill GO and the RaniPill HC. Rani has successfully conducted several preclinical and clinical studies to evaluate safety, tolerability and bioavailability using RaniPill capsule technology. For more information, visit ranitherapeutics.com.
Total Page Views: 933














