Qureight Announces World’s First Digital Biomarker for Lung Fibrosis
Qureight recently presented groundbreaking data at the European Respiratory Society meeting in Barcelona, Spain. The company’s breakthrough has the potential to transform clinical trials involving complex diseases – and, eventually, treatment decisions for patients.
For the first time, artificial intelligence (AI) using convolutional neural networks has been used to analyse trial data involving the lung disease idiopathic pulmonary fibrosis (IPF). The technology picked up differences in response between patients who had an experimental drug compared with those who took a placebo – something that traditional analysis had failed to find.
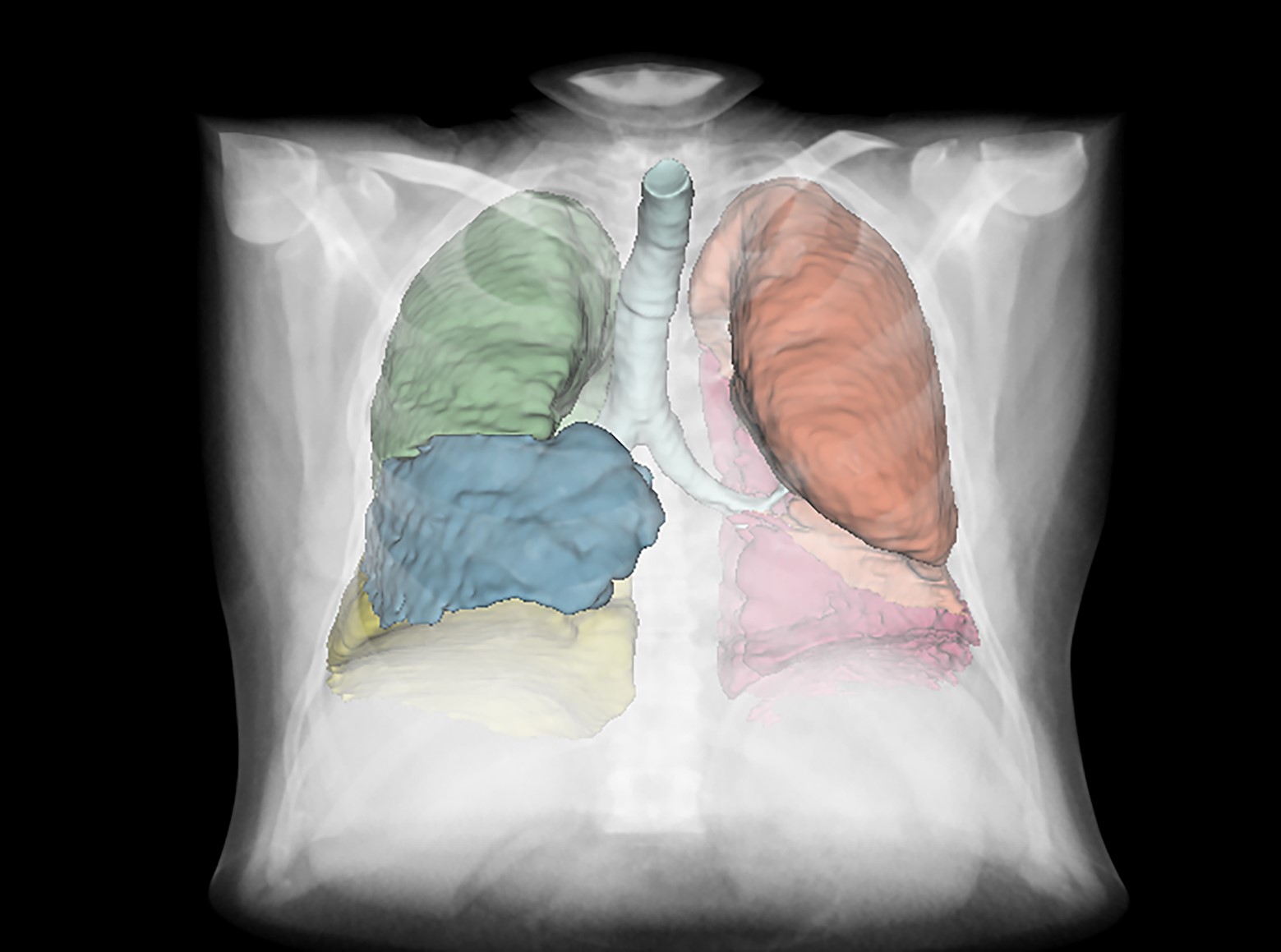
A graphical representation of the Lung8 software which demonstrates the accurate identification and analysis of the different lobes of the lungs.
IPF is a condition in which the lungs become scarred and breathing becomes increasingly difficult. Such scarring can also be a long-term consequence of Covid-19 infection. IPF is difficult to treat, and monitoring requires complex breathing tests in hospital. The test has a number of problems and is not always an accurate measure of lung capacity. Yet in clinical trials to test new drugs for IPF, pharmaceutical companies currently use breathing tests as the main measure of deciding whether a new drug is working or not.
For this latest study, Qureight partnered with Belgian company Galapagos, which was conducting a Phase 2 clinical study across Europe to test a new IPF drug. Called the PINTA trial, it was a double-blind, placebo-controlled study of the drug GLPG1205 in 68 IPF patients. GLPG1205 resulted in smaller declines in FVC (forced vital capacity) and total lung volume at week 26 versus the placebo – but the difference was not significant. After completion of the trial, the data was interrogated with the Qureight platform.
Using its state-of-the-art imaging software called Lung8, Qureight was able to show that CT scans over 26 weeks revealed a significant difference in lung volume change between subjects administered GLPG1205 (+1.51%) versus those administered a placebo (-4.48%) p=0.027. These results support that GLPG1205 slows reduction in lung volume over time in IPF compared with a placebo.
Qureight CEO Dr Muhunthan Thillai, a lung specialist at Royal Papworth Hospital in Cambridge, said “Existing clinical trials involving IPF can cost more than $150 million per drug study and take at least 12 months to look for breathing changes in patients. Our novel platform technology could halve the duration of trials and therefore significantly reduce the costs involved. This will be invaluable for our pharmaceutical partners as they plan their next set of drug trials for this complex disease.”
One of those involved in the groundbreaking study was Professor Phil Molyneaux, director of the National Institute for Health and Care Research (NIHR) Cardiorespiratory Clinical Research Facility at the Royal Brompton Hospital in London. He said: “Pharmaceutical companies have used lung function as the main trial outcome for more than a decade. The Qureight platform is challenging that paradigm – looking for novel endpoints in these trials. The technology has the potential to transform the structuring of clinical trials involving complex diseases like IPF and, eventually, the treatment decisions for our patients.”
Qureight is a Cambridge-based company that uses cloud-based technology to assist in the structuring of image and paired clinical metadata in complex diseases. By structuring data in this way, it allows for the development of AI-based tools to better understand diseases including lung fibrosis, complex cancers and covid-19. For more information, www.qureight.com.
Total Page Views: 915













