Bioavailability & Solubility
GLOBAL REPORT - 2017 Global Drug Delivery & Formulation Report: Part 3, Notable Transactions & Technologies of 2017
In part 3 of this 4-part series, PharmaCircle in collaboration with Drug Development & Delivery, reviews transactions and technologies that provide greater insight into what we can expect in terms of product development and approvals over the next decade.
SOLID FORM SCREENING - Phase Appropriate Strategies for Solid Form Discovery & Development
Pingyun Chen, PhD, describes a rational, fit-for-purpose strategy for solid form screening and selection to ensure a successful yet cost-effective progression of drug candidates from discovery, clinical trials, and commercialization.
INJECTABLE NANOMEDICINES - New Developments in Long-Acting Injectable Nanoformulations
Dongwei Guo, PhD, and Jingjun Huang, PhD, focus on the overview of nanoproducts in the market and the technologies to make long-acting injectable nanoformulations.
MONOCLONAL ANTIBODIES - The Development of Therapeutic Monoclonal Antibody Products: A Comprehensive Guide to CMC Activities From Clone to Clinic
Howard L. Levine, PhD, and Brendan R. Cooney, provide a guide to product development companies, service providers, investors, and analyst as they work their way through the complex and rapidly evolving world of therapeutic monoclonal antibodies.
SPECIAL FEATURE - Challenging Molecules Drive Developers to Get More Creative With Excipients
Contributor Cindy H. Dubin highlights the techniques various excipient manufacturers are using to develop more innovative and effective ingredients to improve the performance of drug molecules.
3D PRINTING - 3D Printed Drugs Hold Great Potential for Personalized Medicine
Contributor Cindy H. Dubin explores 3D printed drugs in the wake of a milestone in the pharma industry when Aprecia Pharmaceuticals’ Spritam (levetiracetam) tablets became the first FDA-approved prescription drug product manufactured using 3D printing technology.
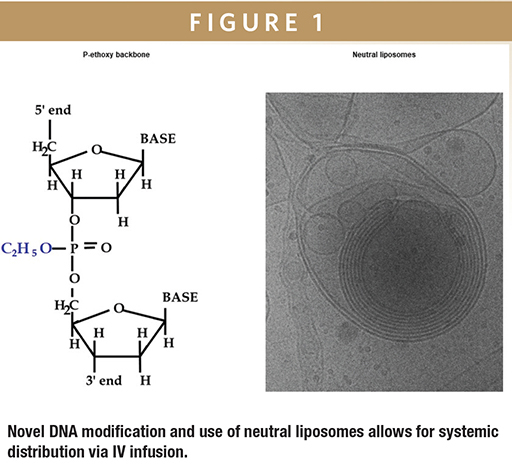
DNA THERAPEUTICS - DNAbilize-ING Antisense
Peter Nielsen, MBA, explains how his company’s candidates are differentiated from those in development at other companies by the type of modification to the antisense molecule and the method by which it is conveyed to its target cell.
EXECUTIVE INTERVIEW - NanOlogy: Submicron Particle Platform Transforms Systemic Chemotherapy Into Local Delivery
Marc Iacobucci, Managing Director of NanOlogy, discusses his company’s technology, clinical program, and efforts to transform cancer therapy.
SPECIAL FEATURE - Improving Bioavailability & Solubility: A Top-Down Versus Bottom-Up Approach
Contributor Cindy H. Dubin speaks with several innovative companies on their science, techniques, and technologies aimed at improving bioavailability and solubility.
NANOMEDICINES - V-Smart® Nanomedicines: Non-Invasive Targeted Brain Therapeutics for CNS Diseases
Susan Rosenbaum, JD, and Irwin Hollander, PhD, present a unique and novel solution to this greatest challenge in medicine for brain disease treatment, considered the “Holy Grail of Neuroscience,” with their breakthrough innovation.
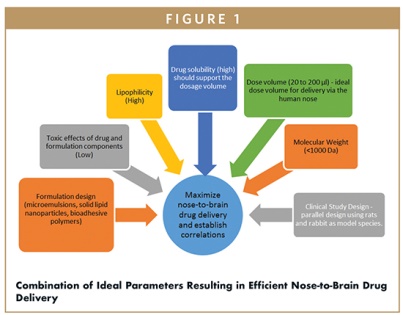
NASAL DELIVERY - A Promising Route of Drug Delivery to the Brain: Scientific Considerations
Vinayak Pathak, MPharm, MBA, indicates after reviewing clinical experiments published in this area, it is evident that formulation design, altering the physico-chemical properties of the drug, addition of absorption enhancers, and mucoadhesive polymers did result in higher bioavailability of drugs in animal models via the nasal route when compared to parenteral administration of the same drug.
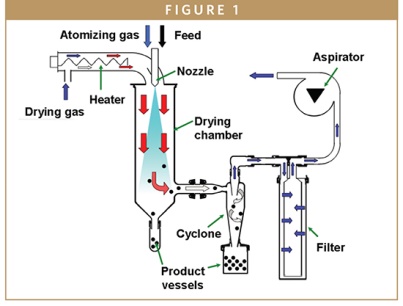
PARTICLE DESIGN TECHNOLOGY - Technical Guide for Solid Dispersion Development
Nathan Barksdale and Elizabeth Hickman, MBA, say there are many articles on the theory and scientific principles underpinning the benefits of ASD, and introduce the reader to the steps involved in the development and manufacturing of an ASD via the spray drying process.
FOAMED SILICONE - Molded Porous Silicone for Delivery of Macromolecules & Low-Solubility APIs
James Arps, PhD, and Matt Petersen, PhD, investigate how foamed silicone is capable of sustained, controlled elution of hydrophobic small molecule and large macromolecular payloads.
ANTIBODY THERAPEUTICS - Teneobio’s Next Generation of Multispecific Antibody Therapeutics
Omid Vafa, PhD, MBA, reviews unique technologies, including a transgenic rat platform expressing human heavy chain antibodies, and a state-of-the-art sequence-based discovery engine, to create novel multispecific antibodies for various therapeutic indications.
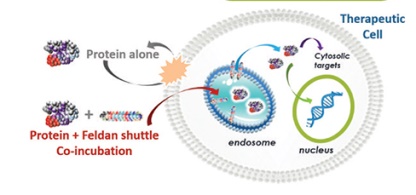
INTRABODY DELIVERY - The Feldan Shuttle Technology: A Peptide-Based Method to Deliver Antibodies
Thomas Del’Guidice, PhD, Nancy Messier, PhD, and David Guay, PhD, present the Feldan Shuttle technology, a peptide-based delivery method that could provide efficient and safe intrabody delivery in mammalian cells.
LIPID-BASED DELIVERY - Are Lipid-Based Drug Delivery Systems in Your Formulation Toolbox?
Jason M. LePree, RPh, PhD, reviews the causes of poor bioavailability for drugs and provides an introduction to lipid-based drug delivery systems, and how the formulation approach can be used to overcome impediments to good bioavailability of therapeutic actives.
EXECUTIVE INTERVIEW - Particle Sciences: Experts in Development & Manufacturing of Complex Dosage Forms
Dr. Mark Mitchnick, CEO of Particle Sciences and CMO of Lubrizol, discusses the rise of complex drug products, the capabilities needed to develop and manufacture these products, and the company’s expansion in this area.
SPECIAL FEATURE - Platform Technologies - Derisking & Transforming Drug Development
Contributor Cindy H. Dubin, in this second annual report, speaks with several exciting and innovative companies whose platform technologies are transforming drug development.
2017 GLOBAL DRUG DELIVERY & FORMULATION REPORT - Notable Technologies, Approvals, Transactions, Pipelines & Perspectives
This third annual report, a collaborative effort between team members at Drug Development & Delivery and PharmaCircle, provides a look back at 2016 in terms of approvals and developments in the area of drug delivery and formulation. The report continues to cover the following significant key points of interest, with the belief that understanding the past, even the recent past, can provide insights to what is possible.
SPECIAL FEATURE - Wanted: New Excipients to Meet the Demands of a Challenging Industry
Contributor Cindy H. Dubin recently spoke with some of the leading excipient innovators to find out what types of excipients they are developing, the advantages they offer to formulations, and where they see the industry focusing throughout the next few years.
Bioavailability and Solubility Challenges
Given that a large number of drugs fail to reach the market due to poor solubility and bioavailability, the industry is seeking various methods to mitigate this challenge while many choose to re-formulate existing product candidates. Either way, the demand for novel bioavailability and solubility enhancement methods has grown significantly. To cater to this increasing demand, many contract manufacturers and technology developers have emerged.
What is Solubility?
Solubility is the ability for a drug to be dissolved in an aqueous medium. Drug solubility is defined as the maximum concentration of a substance that can be completely dissolved in a given solvent at a certain temperature and pressure level.
Solubility of drugs is measured by the amount of solvent needed to dissolve one gram of the drug at a specific temperature. For example, a drug that is very soluble needs less than one part solvent to dissolve one gram of the drug. How soluble a drug is varies widely—a drug that is considered soluble needs 10-30 parts, one that is slightly soluble needs 100-1,000 parts and one that is practically insoluble or insoluble needs more than 10,000 parts. How soluble a drug is depends on the solvent, as well as temperature and pressure.
Since 1975, approximately 60 marketed drugs have leveraged solubilization technologies to enhance oral bioavailability. In the preceding 36 years, from the time the FDA required submission of an NDA in 1938, solubilization technology was virtually unused on a regular basis. Apparently, the disease areas focus, drug discovery methodologies, and the lack of mature solubilization platforms restricted the use prior to the 1970s.
In comparison, the past nearly 4 decades have shown robust growth in the reliance on solubilization platforms, accounting on average for around 9% of all NMEs approved from 1975 through 2022, and more than 10% in the past decade. Some years stand out to validate the need and use of solubilization platforms. For example, in 2005, 20% of NMEs approved used technologies including solid dispersion, lipid, and nanocrystal platforms. The data for the most recent 4-year period (2010-2013) seems to represent a slight decline in growth, but it is still early in the decade, and the data set is relatively small. Based on the trends throughout the past 4 decades and the changing chemical space in drug development, we expect the decade will show additional and significant current growth in use of solubilization technologies once we have visibility into the full 10-year period.
Bioavailability & Solubility Impediments
The biggest impediment in addressing bioavailability issues likely lies with a lack of deep familiarity with enabling technologies. Improving drug bioavailability begins with a thorough evaluation of the API’s physical and chemical properties in relation to solubilization in the dose, but more importantly its dissolution in vivo at the site of absorption.
These technologies, such as nanoparticles, cocrystals, computer-aided prodrug design, and electrospinning, represent innovations aimed at enhancing the solubility of a candidate molecule, particularly in the gastrointestinal tract. Technologies such as electrospinning, deep eutectic solvents, and ionic liquids are upcoming formulation approaches to enhance drug solubility, and as the science matures, and the relative strengths and weaknesses are better understood, we expect to see further application of these innovative approaches. They have shown to be successful for some compounds, and have a place alongside other bioavailability enhancement technologies, where each strategy has its benefits and corresponding liabilities. For them to be successful and widely adopted however, they will also have to provide a compelling benefit compared with other well-understood, and commercially precedented technologies, such as amorphous solid dispersions and lipid-based formulations.
Extreme compounds require either significant amounts of stabilizers to maintain the amorphous state or they are not amenable to common manufacturing technologies with reasonable cost of goods due to their low solubility in organic solvents. These include amorphous solid dispersions using polymethacrylate, cellulose, or povidone-based polymeric carriers, she says. In addition, thermostability of new molecular entities becomes an issue as most new molecules have melting points well above 400°F. Alternative production methods for amorphous solid dispersions can address these issues.