SPECIAL FEATURE - Platform Technologies - Derisking & Transforming Drug Development
Platform technologies have the ability to radically improve upon current products and generate completely novel products. In this sense, they open up new arenas for drug discovery and development, potentially increasing the number of therapeutic options for patients, says Brian Atwood, CEO of Cell Design Labs.
“Once a single compound or therapeutic has been generated and demonstrates a clinical benefit in patients, it is more likely this platform technology can successfully be applied to other therapeutic areas, derisking future compounds/products,” says Mr. Atwood.
Complex drugs by their very nature are challenging and costly to manufacture. This, in turn, translates into higher costs for patients and other payers, explains Ross Macdonald, PhD, Managing Director and Chief Executive Officer of Cynata Therapeutics. He says: “In order to provide safe and effective therapies at a reasonable price, it is necessary for the industry to develop manufacturing technologies that reduce costs and provide a consistent product.”
“While the initial investment may be larger, manufacturing costs will be lower over time as the manufacturing process is solidified,” agrees Mr. Atwood.
Despite the initial upfront costs, Dr. Macdonald says that platform technologies inevitably provide pragmatic solutions to production challenges, while yielding safer and more effective therapeutic products.
Drug Development & Delivery’s second annual report on platform technologies highlights some of these novel discoveries and describes how they are transforming drug development.
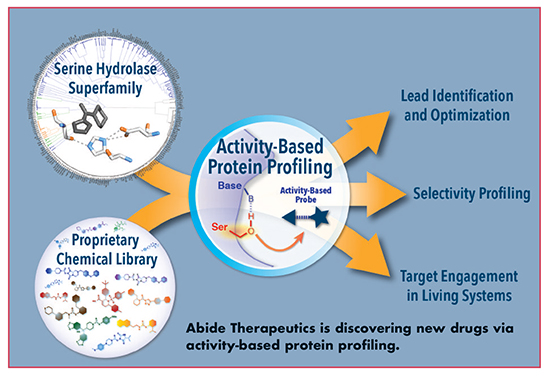
Abide Therapeutics: A Transformative Platform for Serine Hydrolase Drug Discovery
Abide Therapeutics integrates a powerful chemoproteomic technique called activity-based protein profiling (ABPP) with innovative chemistry to develop first-in-class medicines targeting a large family of enzymes – the serine hydrolases – that plays key roles in physiology and disease, explains Alan Ezekowitz, MBCHB, D.PHIL., FAAP, President and CEO of Abide.
“The serine hydrolase superfamily is one of the largest known enzyme classes, comprising approximately 250 enzymes,” he says. “These enzymes are involved in a range of physiological processes, including CNS signaling, digestion, inflammation, and metabolism. Several approved drugs (Xenical®, Januvia®, Aricept®) have shown that the enzyme superfamily is a potentially rich source of first-in-class medicines, yet the value of serine hydrolases remains largely untapped. Abide’s unique platform can fulfill this promise.”
Today, most serine hydrolases are poorly characterized, but their roles in disease are under active investigation with Abide’s chemical library, which is tailored to selectively target these enzymes in live cells and animals, providing a means to concurrently validate new drug targets and discover new inhibitors. Abide’s ABPP technology enables precise measurement of enzyme activity through the use of broad-spectrum chemical probes that covalently react with the active sites of serine hydrolases, all of which rely on a shared catalytic mechanism. New drugs can be discovered via ABPP by allowing small molecules from Abide’s library to compete with activity-based probes for binding to enzyme active sites, thereby inhibiting probe labeling, explains Dr. Ezekowitz.
“This technique has several advantages over classical approaches for measuring drug activity,” he says. First, enzymes can be screened in their native context, within cells and tissues, without requiring recombinant expression or purification of each target. Second, the activity-based probe serves as a “universal” assay for its enzyme targets, meaning that inhibitors can be developed for enzymes that have no known substrates or biomarkers. Third, inhibitors are screened against many enzymes in parallel, enabling concurrent optimization of inhibitor potency and selectivity.
“Abide’s unique approach provides a deep understanding of inhibitor action in living systems before moving to human testing, assuring drug candidates reach their intended targets in the physiologically relevant tissue while sparing off-targets that could lead to detrimental side effects,” says Dr. Ezekowitz.
Using this transformative platform, Abide brought its first drug candidate from the lab to the clinic in less than three years. ABX-1431 is a first-in-class inhibitor of the serine hydrolase monoacylglycerol lipase (MGLL) designed to treat nervous system disorders by tuning brain signaling networks in an unprecedented way. A study of ABX-1431 in patients with Tourette Syndrome is ongoing, and other CNS disorders, such as multiple sclerosis, are being investigated.
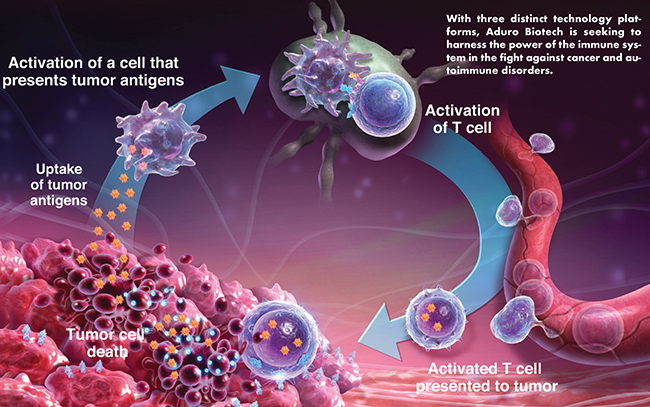
Aduro Biotech: Harnessing the Power of the Immune System Through Three Diverse Technology Platforms
With three proprietary immunotherapy platforms — Live, Attenuated Double-Deleted Listeria (LADD); STING Pathway Activators; and B-select monoclonal antibodies — Aduro Biotech is positioned to transform the treatment of challenging diseases, including cancer, says Andrea van Elsas, Chief Scientific Officer of Aduro Biotech.
Aduro’s LADD platform harnesses the power of bacteria to direct the immune response against cancer. The platform is based on proprietary attenuated strains (strains in which two virulence genes have been deleted) of Listeria monocytogenes that have been genetically engineered to express specific tumor antigens. Aduro is also leveraging this technology to develop a personalized approach to cancer treatment, which uses tumor markers from a patient’s own tumor cells. To date, LADD product candidates have been administered to more than 350 patients and have been generally well-tolerated, says Dr. van Elsas.
Aduro’s STING Pathway Activators platform seeks to use a patient’s own tumor composition to activate an anti-cancer immune response. This approach is based on the activation of the STING (Stimulator of Interferon Genes) receptor, which is generally expressed at high levels on immune cells. “The company’s proprietary STING pathway activator product candidates, including ADU-S100 (MIW815), are synthetic small-molecule immune modulators designed to target and activate human STING, thereby initiating a profound innate immune response through multiple pathways,” Dr. van Elsas says. These synthetic STING Pathway Activators are being developed for direct injection into tumors to stimulate an immune response against antigens present in the tumor.
“The process is expected to use the tumor itself as a vaccine, enabling the induction of a tumor-specific immune response that is unique to the treated individual — an off-the-shelf small molecule approach to personalized immunotherapy,” he says.
Aduro’s B-select antibody platform seeks to modulate cancer immunity. The company’s propriety, ultra-selective screening process is designed to identify antibodies with unique binding properties against a broad repertoire of targets. Through this process, Aduro has developed a deep pipeline of proprietary monoclonal antibodies (mAbs) that hold the potential to regulate the immune system in the fight against cancer. Two investigational mAb product candidates in Aduro’s pipeline include BION-1301, a novel anti-APRIL antibody, and an anti-CD27 antibody being developed in partnership with Merck.
Cell Design Labs: Disruptive Biology Platform for Cell-Based Oncology Therapies
Cell Design Labs is a biotherapeutics company leveraging the power of the body’s immune system to develop smart living therapies for patients with cancer. “Currently, modified immune cells – CAR T-cells – are poised to revolutionize the field of cancer therapy,” says Brian Atwood, CEO and Co-founder of Cell Design Labs. The most commonly used approaches to generating engineered immune cells are modifying T-cells with chimeric antigen receptors (CAR-Ts) or with tumor antigen-specific T-cell receptors (TCR-Ts). However, Mr. Atwood points out that excitement for these new therapeutics is tempered by three primary challenges: safety, including off-target toxicity; development of resistance; and a lack of clinical efficacy in solid tumors. Cell Design Labs’ customized molecular modules, THROTTLE SwitchTM and synNotchTM engineered modules, enable design of next-generation CAR-Ts and TCR-Ts to overcome these challenges.
synNotch modules: By modifying the external and internal portions of the naturally occurring Notch receptor, and placing these unique constructs into a patient’s immune cells, the patient’s body can be instructed to detect new molecular targets and to turn on new genes. In addition, further modifications of the synNotch scaffold can enable diverse sensing and response behaviors. By expressing synNotch receptors in T-cells, it is possible to create a programmable immune cell. When this reprogrammed cell binds to its sole intended target (ie, a cancer cell), it triggers one or more specific molecular activities: locally producing and modulating tumor defense mechanisms such as a specific CAR, delivering drugs such as checkpoint inhibitors, inducing a customized cytokine profile to supercharge the immune system, or encouraging T-cells to differentiate into specific subtypes to convey long-term protection against cancer recurrence. “With this approach, the synNotch receptor confers extremely versatile sense-and-response functionality to T-cells, which already have the ability to migrate throughout the body to find targets,” Peter Emtage, PhD, Chief Scientific Officer of Cell Design Labs, says.
THROTTLE Switch modules: The company’s proprietary THROTTLE Switch technology allows CAR-T cells to be turned on and off, enabling them to be better tolerated and more effective, says Dr. Emtage. This is achieved by constructing two separate and inactive receptor components into the cell membrane of a CAR-T cell, meaning the natural state of the CAR-T cell is “off.”
“Only in the presence of an FDA-approved small molecule compound will the two come together – closing the “switch” – into an active state, seeking out and eliminating cancer cells,” says Dr. Emtage. “With this type of control-switch CAR-T cells, physicians can rapidly and precisely control the activity and safety of CAR-T cells.”
Mr. Atwood adds: “Cell Design Labs is operating at the edge of scientific discovery to harness fresh insights about cell behavior and computer-based modeling to create a new generation of medicines. While currently focused on cancer, this platform may have broad applicability to a number of serious diseases.”
Cynata Therapeutics: Technology Platform Could Revolutionize Regenerative Medicine
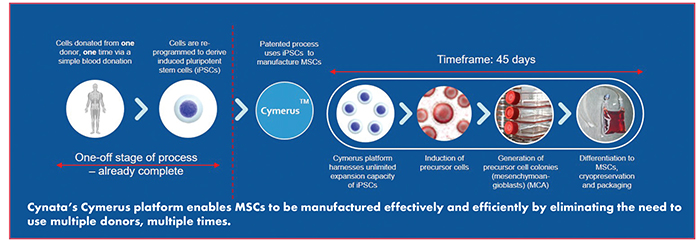
“Cynata Therapeutics’ proprietary CymerusTM technology platform has the potential to revolutionize the regenerative medicine industry and overcome one of its biggest obstacles: the economical manufacture at commercial-scale of mesenchymal stem cells (MSCs) for therapeutic use,” says Ross Macdonald, PhD, Managing Director and Chief Executive Officer of Cynata Therapeutics.
MSCs are multipotent cells found in a variety of human tissues. Dr. Macdonald says there is significant interest in MSCs’ therapeutic potential across many diseases, due to their immunoregulatory and tissue regeneration properties. “In fact, MSCs are often called medicine-secreting cells because of their impressive production of bioactive molecules.”
There are more than 600 trials worldwide investigating the potential clinical utility of MSCs in conditions like cardiovascular disorders, stroke, and autoimmune diseases. Product for these trials is obtained using first-generation production techniques that require repeat donor-derived materials and massive expansion of MSCs in culture to derive sufficient quantities. “These production techniques have significant limitations, most notably, the small number of MSCs recovered from each donation and limits on MSC expansion,” says Dr. Macdonald.
Cynata’s Cymerus technology may overcome these issues by generating large-scale MSCs based on a single blood donation from one adult donor. Cynata’s novel approach involves using induced pluripotent stem cells (iPSCs) as starting material. iPSCs are derived by reprogramming the donor’s cells from a single donation. iPSCs can reproduce indefinitely without losing their pluripotent characteristics, thereby providing an essentially limitless and consistent source for the manufacture of the finished product, the MSCs, via a unique intermediate cell type called mesenchymoangioblasts (MCAs).
Cymerus mitigates concerns associated with batch-to-batch variability and excessive expansion in culture, and eliminates the need to repeatedly identify new donors. A typical bone marrow donation yields 10,000–20,000 MSCs1,2, though clinical doses are usually 35–350 million.3 Therefore, first-generation methods may require hundreds, if not thousands, of donations each year to meet commercial demand. In addition, switching donors requires robust comparability testing to ensure a consistent clinical outcome is derived from the different donations. Dr. Macdonald points out that it cannot be assumed that MSCs expanded at a large scale will retain the same functional properties. It has been suggested that MSCs expanded to produce 10,000 doses per donation may have limited efficacy versus modestly expanded MSCs.4
“The Cymerus process produces an essentially unlimited number of new MSCs,” says Dr. Macdonald. “Moreover, Cymerus MSCs originate from one donor and are identical to one another, limiting the risk of contaminating non-target cells and eliminating the need for donor-to-donor comparability.”
Cynata is conducting the world’s first clinical trial of allogenic iPSCs-derived MSCs. The ongoing trial of CYP-001 in graft vs. host disease (GvHD) is enrolling in the UK and Australia, and is expected to be completed by early 2018. Cynata’s positive pre-IND meeting with the FDA in May 2017 cleared the path for a U.S. clinical program. In addition, preclinical data generated by Cynata’s academic partnerships support expansion into diseases like asthma5, heart attack6, and glioblastoma.
Heat Biologics: Making an Impact on Immunotherapy
Heat Biologics is a biopharmaceutical company developing immunotherapies designed to activate a patient’s CD8+ “Killer” T-cells against cancer. “We believe the future of cancer immunotherapy will be focused on drug combinations, and will lead to the success of finding effective and lasting treatments for patients with cancer,” says Jeff Hutchins, PhD, Chief Scientific Officer, Heat Biologics.
Heat uses its Immune Pan-antigen Cytotoxic Therapy (ImPACT®) platform to develop product candidates that consist of live, allogeneic “off-the-shelf” genetically modified, irradiated human cancer cells. These cells are intended to secrete a broad spectrum of Cancer Testis Antigens (CTA), classified as tumor antigens, together with the “heat shock” gp96 protein.
Through ImPACT, Heat developed HS-110 as a potential treatment for patients with non-small cell lung cancer (NSCLC). It is the company’s first biologic product candidate in a series of proprietary ImPACT-based immunotherapies designed to stimulate a patient’s own T-cells to attack cancer. HS-110 is made of a cancer cell line that has been genetically modified, and is designed to secrete a range of lung cancer-associated antigens bound to gp96 proteins, to activate and expand a broad, T-cell-mediated immune response against the patient’s cancer, explains Dr. Hutchins.
The T-Cell Activation Platform (TCAP) approach produces therapies designed to turn immunologically “cold” tumors “hot,” and be administered in combination with checkpoint inhibitor therapies and other immuno-modulators to increase their effectiveness. This approach can also be used to combine with existing T-cell checkpoint inhibitors and co-stimulators in a single treatment. This offers the potential benefit of combination immunotherapy without the need for multiple, independent biologic products.
“Unlike autologous or “personalized” therapeutic, monotherapy approaches that either require the extraction of blood or tumor tissue from each patient or the creation of an individualized treatment, the product candidates are fully allogeneic, and do not require extraction of individual patient’s material or custom manufacturing,” he says. “As a result, the products can be mass-produced and readily available for immediate patient use. Because each patient receives the same treatment, Heat’s immunotherapy approach offers logistical, manufacturing, and other cost benefits, compared to patient-specific or precision medicine approaches.”
Idera Pharmaceuticals: Improving the Safety & Efficacy Profile of Proprietary Oligonucleotide Therapeutics
Antisense oligonucleotides hold the promise for targeting diseases caused by aberrant RNA that are considered “undruggable” by traditional therapeutic plat-forms. This technology has been in development for a few decades, with tremendous progress made in the pharmacokinetic and pharmacodynamics properties of oligonucleotides. Recent regulatory approvals bring the hope that the technology is nearing maturity. Nevertheless, broad applicability of antisense therapeutics has been hampered by factors such as pro-inflammatory effects, tissue build-up leading to toxicities, and off-target effects, explains Jonathan Yingling, PhD, Idera’s Head of Early Development.
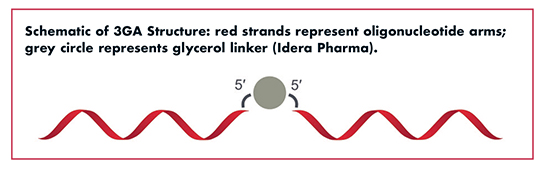
Idera Pharmaceuticals is a clinical-stage biopharmaceutical company developing nucleic acid therapeutics for the treatment of certain cancers and rare diseases. Idera has focused its research efforts on understanding not only gene silencing technology, but also the interaction of oligonucleotides with the host immune system. “The latter provides critical insight for addressing factors that continue to limit the therapeutic index of gene silencing oligonucleotides,” says Dr. Yingling. “Based on our knowledge and expertise in nucleic acid therapeutics and Toll-like receptor biology, we have designed novel gene silencing oligonucleotides, called third generation antisense oligonucleotides (3GA).”
3GAs are composed of two short oligonucleotides complementary to target RNA, linked via their 5’-ends with a glycerol linker. “The elimination of free 5’-ends mitigates immune activation while the resulting exposure of the 3’-ends facilitates nuclease degradation, thereby reducing tissue build-up,” explains Dr. Yingling. “This design can also lead to improved gene silencing potency.”
In parallel, Idera has created a proprietary algorithm for optimized target sequence selection. This algorithm, combined with the unique 3GA structure and the flexibility to introduce positional modifications to optimize individual 3GA assets, provides the foundation for applying the platform across various targets and diseases.
Early mechanistic studies have shown that the central region of the 3GA is critical for target engagement. “Indeed, incorporation of a single nucleotide mismatch at the central region of the 3GA leads to reduction in gene silencing activity, suggesting that 3Gas can be highly specific,” Dr. Yingling says. “We are currently leveraging this cleavage site specificity to design 3GAs that can selectively target point mutations. More detailed mechanistic studies are underway to further understand how 3GAs work at the molecular level. These studies, as well as other research efforts, inform our strategy and design as we continually work to improve the safety and efficacy profile of proprietary oligonucleotide therapeutics.”
Mannin Research Inc. & Q Biomed Inc.: Small Molecule That Activates the Angiopoietin-TIE2 Signaling Pathway
Mannin Research is a biotechnology company focused on the discovery, development, and commercialization of first-in-class therapeutics for vascular diseases, utilizing the angiopoietin/TIE2 signaling pathway. Mannin Research partnered with Q BioMed Inc., a biotech acceleration company, that provides strategic resources to accelerate the development of biotech assets.
TIE2 is a transmembrane receptor tyrosine kinase. The primary ligands for the receptor are angiopoietin-1 (ANGPT1) and angiopoietin-2 (ANGPT2); two protein growth factors that promote angiogenesis and the formation of blood vessels. TIE2 is expressed almost exclusively in endothelial cells in mice and humans. The angiopoietin/TIE2 (ANGPT/TIE2) signaling pathway is a major regulator of vascular development and homeostasis, and altered expression of ANGPT ligands or activity of the TIE2 receptor is linked to a variety of vascular diseases and adverse outcomes in patients.
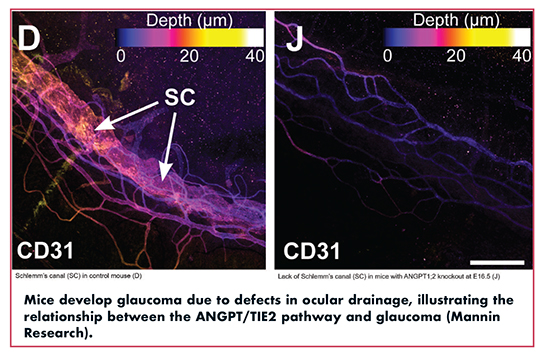
Mannin is currently developing small molecules targeting the ANGPT/TIE2 pathway designed to reduce intraocular pressure (IOP) and treat primary open-angle glaucoma. In an article in the Journal of Clinical Investigations, Dr. Susan Quaggin, Mannin Research’s Chief Scientific Officer, and Director of the Feinberg Cardiovascular Research Institute and Chief of Nephrology and Hypertension in the Department of Medicine, demonstrated that genetic disruption of the angiopoietin/TIE2 signaling pathway results in high IOP, buphthalmos, and classic features of glaucoma, including retinal ganglion degeneration and vision loss.
Dr. Quaggin explains that the angiopoietin/TIE2 signaling pathway is a key signaling pathway that is essential for proper drainage and maintenance of intraocular pressure, and is needed for proper growth and function of the drainage apparatus in the front of the eye. A specialized blood vessel, known as Schlemm’s canal, is the major drainage pathway for fluid to escape from the eye. Blockage of this canal or a birth defect resulting in a small or absent Schlemm’s canal, leads to glaucoma in patients. The loss of angiopoietins 1 and 2 also results in deterioration or absence of the Schlemm’s canal.
She says that to treat glaucoma, it is necessary to increase the drainage of fluid from the front of the eye, preferably by way of a novel eye-drop treatment. This can be accomplished by increasing the flow of fluid through Schlemm’s canal. “To that end, Mannin is in lead optimization of a small-molecule therapeutic that will activate the Angiopietin-TIE2 pathway. Mannin’s goal is to develop a painless and simple eye drop therapeutic for patients with glaucoma,” says Dr. Quaggin.
Although Mannin Research is currently focused on developing a therapeutic for glaucoma with technology partner Q BioMed as its first indication, Mannin and Q BioMed believe the technology can be applied to treat various vascular-related diseases, such as cystic kidney disease, Ebola, cardiorenal syndrome, congestive heart failure, and more.
Maxcyte: Chimeric Antigen Receptor Uses Messenger RNA to Engineer Immune Cells
MaxCyte has developed a novel and proprietary platform for next-generation chimeric antigen receptor (CAR)-engineered T/NK-cell therapies (CAR-T). CARMA, as a CAR platform, provides several unique attributes over existing CAR-T products and platforms, according to Doug Doerfler, President and CEO of MaxCyte.
First, CARMA is differentiated from traditional CAR-T therapy by its use of messenger RNA (mRNA) to engineer the immune cells that are delivered back into a patient, without the need for a viral component. CARMA provides for transient expression, as shown in preclinical studies, to control the adverse side effects seen in first-generation, viral-based CAR therapies. This allows for CAR-T immunotherapies to address a broader range of cancers, including solid cancers, than traditional CAR approaches.
Second, CARMA offers the potential to deliver therapy to the patient in a fraction of the time of other autologous CAR-T products, says Mr. Doerfler. This is due to a more streamlined manufacturing process without the complexity of virus-based products. MaxCyte’s first CARMA drug candidate is moving towards clinical development in a solid tumor indication, where CAR-based treatments have been less successful. This product candidate, MCYM11, employs repeat infusions of mesothelin-specific mRNA CAR-transfected into PBMCs (peripheral blood mononuclear cells) to permit controlled persistence, which other CAR-T therapies are seeking, says Mr. Doerfler. Early non-clinical testing has provided preliminary evidence of the safety and anti-tumor activity of this platform. MaxCyte and its collaborators at the Johns Hopkins Kimmel Cancer Center explored the various attributes of MCY-M11. The studies found that cryopreserved MCYM11 expressed CAR in >95% of cells, and recognized and lysed tumor cells in an antigen-specific manner. In addition, expression of CAR was detectable for 5 to 7 days in vitro, with a progressive decline of CAR expression related to in vitro cell expansion, Mr. Doerfler explains.
In a murine ovarian cancer model, a single intra-peritoneal (IP) injection of CARMA product resulted in the dose-dependent inhibition of tumor growth and prolonged the overall survival (OS) of the mice. Weekly IP injections of the optimized MCY-M11 dose boosted disease control and further prolonged OS, both of which improved with an increasing number of injections. No significant off-tumor toxicities were observed.7
“The data provide a strong scientific foundation for clinical development of our novel and proprietary CARMA platform providing ‘controlled persistence’ in tumor-targeted immunotherapies using peripheral blood lymphocytes,” says Mr. Doerfler. “We can control ‘on-target, off-tumor’ toxicities, while reducing manufacturing complexity for therapies targeting a wide variety of malignancies.”
Pluristem: 3D Macrocarrier Bioreactor Technology Overcomes Limitations of 2D Microcarriers
Pluristem’s products are placenta-derived adherent stromal cells. Placenta-derived cell therapies are allogeneic in nature and are intended to be manufactured in commercial quantities for off-the-shelf use. To fully harness the potential for commercial manufacturing of allogeneic cells, process scalability should involve scale-up and scale-out aspects.
“Most of today’s cell therapy production platforms are based on 2D technologies, such as cell factories or cell stacks,” says Lior Raviv, Vice President Development, Pluristem. “The major drawbacks of these technologies are limits in the total number of cells that can ultimately be produced, significant increases in cost of production, and poor control over culture parameters, which can lead to batch-to-batch variability. Due to the limitations of the standard 2D technologies, some companies use microcarrier-based bioreactor systems to grow their product.”
Microcarrier platforms are considered more like 2D rather than 3D platforms due to the substantial difference between the cell size and the carrier curvature. Although this system is more controlled than common 2D systems, the real manufacturing challenge consists of the shear forces acting on the cells during culturing and harvesting, which compromise cell yield and quality, explains Mr. Raviv.
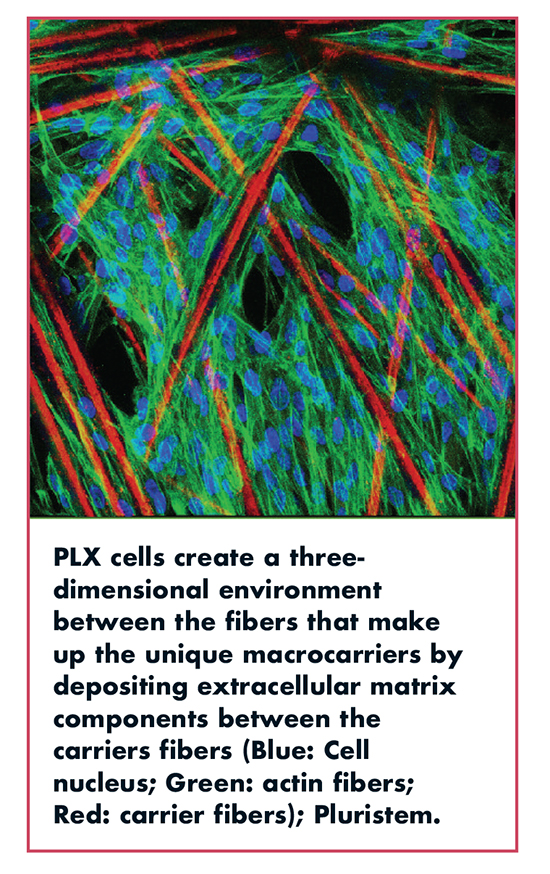
Pluristem developed a bioreactor platform that uses macrocarriers (Fibra-cel Discs) in a packed bed configuration. The Fibra-Cell discs are composed of nonwoven polyester fibers creating a 3D weblike environment, enabling a fast cell attachment (more than 90% in less than 1 hour). During the seeding of cells onto carriers, cells attach to the fibers and start secreting Extra Cellular Matrix components between the fibers, increasing the surface area available for cell growth and creating a 3D environment that is more like these cells’ natural environment.
The combination of the unique growth environment in a packed bed with a specially designed impeller provides shielding from shear forces during growth, protecting the cell quality. Furthermore, in order to have a complete manufacturing solution, Pluristem developed an automated bioreactor harvesting device that controls harvesting parameters and can adjust them to different products. “Our system provides high cell recovery while maintaining cell quality,” says Mr. Raviv. “Our bioreactors also enable automated monitoring and adjustments of physical and chemical factors that can affect cell growth, such as pH, dissolved oxygen, and temperature, thereby reducing batch-to-batch variation to levels not achievable by traditional platforms.”
Precision NanoSystems: Controlled, Tuned & Fully Scalable Manufacturing of Nanomedicines
Nanoparticle encapsulation is gaining momentum as an effective drug delivery system, but traditional manufacturing methods are labor intensive, hard to reproduce, and difficult to scale up. Precision NanoSystems Inc. has developed a proprietary technology – the NanoAssemblrTM platform – for the rapid development of nanoparticles and seamless scale-up for clinical studies and commercial production.
This cartridge-based microfluidic system takes advantage of the highly predictable, time-invariant mixing offered by laminar flow to ensure rapid, controlled production of a variety of nanoparticles for the delivery of drugs or biological molecules.
“This standardization of processing allows the manufacture of nanoparticles with carefully defined characteristics, including chemical compositions, concentrations and drug/excipient ratios,” says James Taylor, CEO and Co-founder.
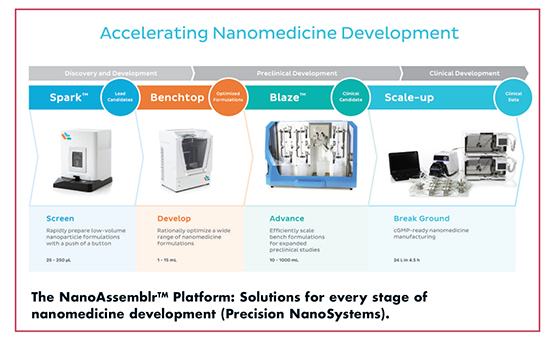
Parallelization is at the core of the NanoAssemblr technology, enabling throughput to be increased to meet the requirements of each phase of the drug development workflow without changing the reaction conditions. Precision NanoSystems offers four small footprint systems based on the same underlying microfluidic technology to match the various needs of the market. The smallest system in the family – the NanoAssemblr SparkTM – is designed for early-stage research and development activities, using disposable microfluidic cartridges to provide controlled and reproducible formulation of 25-250μl nanoparticle suspensions in less than 10 seconds. Once screening is complete, the NanoAssemblr BenchtopTM enables process development and optimization in 1-15-ml volumes. A simple, semi-disposable microfluidic cartridge offers flow rates up to 18 ml/min, and most nanoparticle formulations are completed in just 15-20 seconds, allowing more than 40 formulations to be prepared in one day, explains Mr. Taylor.
“The NanoAssemblr Blaze is designed to seamlessly scale NanoAssemblr Benchtop formulations up to one liter volumes. Using the same microfluidic mixer as the NanoAssemblr Benchtop, it employs continuous flow and parallelization to increase volumes and throughput, greatly reducing the need for process development compared to traditional methods,” says Mr. Taylor.
Finally, the largest system in the family – the NanoAssemblr Scale-Up – has been developed specifically for manufacturing clinical grade materials in the cGMP environment. Designed to be run in a clean room and customizable to meet customers’ specific requirements, it offers a completely flexible solution from early stage clinical trial manufacturing to commercial production. Mr. Taylor says: “This powerful technology is transforming the development and manufacturing of a range of nanoparticle formulations from a hit-and-miss affair to a standardized process, accelerating novel nanomedicines from the bench to the clinic.”
References
1. Ikebe C, Suzuki K. Mesenchymal stem cells for regenerative therapy: optimization of cell preparation protocols. Biomed. Res. Int. doi:10.1155/2014/951512 (2014) (Epub ahead of print).
2. Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu. Rev. Pathol. 6, 457-478 (2011).
3. Herrmann RP, Sturm MJ. Adult human mesenchymal stromal cells and the treatment of graft versus host disease. Stem Cells Cloning 7, 45-52 (2014).
4. Galipeau J. The mesenchymal stromal cells dilemma-does a negative Phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy 15, 28 (2013).
5. Royce, S. Intranasal administration of mesenchymoangioblast-derived mesenchymal stem cells abrogates airway fibrosis and airway hyperresponsiveness associated with chronic allergic airways disease. FASEB J. doi:10.1096/fj.201700178R (2017) (Epub ahead of print).
6. “Positive Preliminary Data from Preclinical Heart Attack Study with Cynata’s MSCs” (2/2/2017).
7. Development of anti-human mesothelin chimeric antigen receptor (CAR) messenger RNA (mRNA) transfected peripheral blood mononuclear cells (CARMA) for the treatment of mesothelin-expressing cancers, April 4, 2017,http://www.abstractsonline.com/pp8/#!/4292/presentation/6025.
Total Page Views: 7577