Market News & Trends
Novavax Initiates Pivotal Phase 3 Clinical Trial
Novavax, Inc. recently announced the initiation of a pivotal Phase 3 clinical trial for NanoFlu, its recombinant quadrivalent seasonal influenza vaccine candidate, in adults aged…
Orgenesis Announces Co-Development Agreement With Accellix
Orgenesis Inc recently announced it has entered into a co-development agreement with Accellix, Inc. The agreement will enable Orgenesis to integrate Accellix’s proprietary optic system, cartridges, reagents,…
An End to Alzheimer’s? New Drugs Fight Memory Loss
Researchers in Toronto have developed an experimental drug that appears to renew the underlying brain impairments causing memory loss, fuelling hopes that a treatment for…
Patient-Derived Tumor Organoid Drug Development Platform Launched
Crown Bioscience recently announced the launch of a new tumor organoid drug development platform with the potential to significantly improve the……
White Paper: Control of Beta-Glucans & Endotoxin in High Purity Sucrose for Biopharmaceutical Applications
This white paper discusses a Quality by Design (QbD) scenario that allows end users to rationally select the well characterized excipients during biopharmaceutical development and develop a control strategy for biologic drug products.
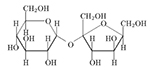
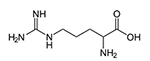
White Paper: High Purity Low Endotoxin Arginine: Applications in Biopharmaceutical Processing & Biotherapeutic Stabilization
This white paper discusses arginine, also known as L-arginine (symbol Arg or R), a basic amino acid widely used in biopharmaceutical processing and stabilization of biotherapeutics.
Credence MedSystems Awarded Gates Foundation Grant to Develop Dual Chamber Drug Delivery Device
Credence MedSystems, an innovator in injectable drug delivery technology for the biopharmaceutical industry, recently announced it has been awarded a grant from the Bill & Melinda Gates Foundation to support the…..
Pfenex Receives US FDA Approval for PF708 to Treat Osteoporosis
Pfenex Inc. recently announced the US FDA has approved the new drug application (NDA) for PF708 submitted under the 505(b)(2) regulatory pathway, with…
Increased Security for Connected Medical Devices to Drive Cybersecurity Market to $143 Billion
The Food and Drug Administration (FDA) recently released a safety communication regarding a set of 11 cybersecurity vulnerabilities, referred to as the URGENT/11, which could…
Sienna Biopharmaceuticals Files 510(k) Submission With FDA
Sienna Biopharmaceuticals, Inc. recently announced the company has filed a Premarket Notification 510(k) submission to the US FDA for SNA-001, a topical pre-treatment to standard…
Stevanato Group Signs License Exclusive Agreement With Haselmeier
Stevanato Group, an Italy-based producer of glass primary packaging and global leader in providing integrated capabilities and solutions for combination products, and Haselmeier, a Switzerland…
MilliporeSigma Becomes the First to Use Acoustic Technology for Cell Therapy Manufacturing
MilliporeSigma recently announced it has acquired FloDesign Sonics, of Wilbraham, MA, developer of a unique acoustic cell processing platform for the industrialization of cell and…
Akcea & Pfizer Announce $250-Million Licensing Agreement for Investigative Therapy
Akcea Therapeutics, Inc., a majority-owned affiliate of Ionis Pharmaceuticals, Inc., and Pfizer Inc. recently announced the companies have entered into a worldwide exclusive licensing agreement…
Bormioli Pharma Completes Acquisition of Remy & Geiser
Bormioli Pharma S.p.A. recently announced the successful completion of its acquisition of Remy & Geiser GmbH (R&G), a German company active in the business of…
Streamlining Drug Development Processes Through the Accenture INTIENT Clinical Platform
To facilitate the demand for this new world order, we’ve unveiled the Accenture INTIENT Clinical platform. It helps companies run faster clinical trials with better transparency and….
Gerresheimer Opens US Glass Innovation & Technology Center
Gerresheimer is driving innovation in pharmaceutical glass, primary packaging glass products, technologies, and digitized processes. In the future, highly qualified engineers at the recently opened Gx Glass Innovation and Technology Center will develop….
Cue Biopharma Doses First Patient in Clinical Trial
Cue Biopharma, Inc. recently announced it dosed the first patient in a Phase 1 clinical trial of CUE-101 at Washington University, Alvin J. Siteman Cancer Center, St. Louis, Missouri for the treatment of….
VivaLNK Delivers Continuomics Using Wearable Medical Sensors
VivaLNK, a leading provider of connected healthcare solutions, is improving clinical trials with continuomics by using wearable medical sensors for….
Cabaletta Bio Receives IND Clearance From FDA to Initiate First Clinical Trial
Cabaletta Bio, Inc. recently announced it has received clearance of its IND application from the US FDA to initiate a first-in-human clinical trial of…..
Thermo Fisher Scientific Expands API Manufacturing Capabilities
Thermo Fisher Scientific recently announced the expansion of its API manufacturing capabilities following the completion of its previously announced acquisition of an active pharmaceutical ingredient…