White Paper: Control of Beta-Glucans & Endotoxin in High Purity Sucrose for Biopharmaceutical Applications
Sucrose or saccharose is a disaccharide composed of glucose and fructose. Figure 1, depicts the chemical structure of sucrose. It has been used in protein stabilization particularly biotherapeutics since the past three decades. The study by Lee and Timasheff, probes the mechanism of stabilization of proteins by sucrose (1). Several other studies besides the fundamental study by Lee and Timasheff (1) have evaluated the stabilization mechanism of sucrose (2-4). These fundamental studies highlight the importance of utilizing thermodynamic stabilization approach for protein pharmaceuticals. Besides they highlight the use of conformational stabilizer like sucrose which acts by the preferential exclusion mechanism.
THERAPEUTIC ANTIBODY FORMULATION COMPOSITIONS STABILIZED USING SUCROSE
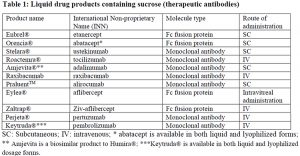
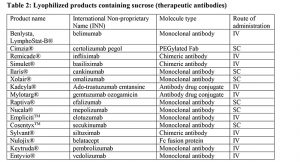
Several antibody formulations that have been approved for therapeutic indications have been stabilized by sucrose (Table 1 and 2). As observed, sucrose has been used to stabilize a diverse set of antibody products including human antibodies, humanized antibodies, chimeric antibodies, Fc fusion proteins and antibody drug conjugates (ADC). Sucrose has been successfully used in both liquid and lyophilized products.
APPROVED PROTEIN THERAPEUTICS (NON-ANTIBODY THERAPEUTICS) STABILIZED USING SUCROSE
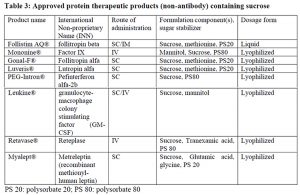
Besides antibody modalities, sucrose has been successfully employed in formulation and stabilization of other non-antibody type biotherapeutics including cytokines, hormones and blood clotting factors. It is due to the conformational (thermodynamic) stabilization property that sucrose can serve as a universal stabilizer for such diverse protein therapeutics.
CONSIDERATIONS FOR USE OF SUCROSE IN BIOPHARMACEUTICAL FORMULATIONS
Sucrose, among other disaccharides (trehalose) and polyols (sorbitol, mannitol) is a preferred stabilizer in lyophilized products since it stays amorphous during several freezing (or freeze-drying) steps. Several studies highlight this property and compare the behavior of sucrose with other polyols (5-8). Sucrose is a component of several platform formulation approaches used for protein formulations (9, 10). While sucrose is widely used for stabilization of liquid protein formulations as well, acid catalyzed sucrose hydrolysis could generate glucose reducing sugar at higher temperatures (11, 12). In a study evaluating the effect of sucrose hydrolysis on the stability of protein therapeutics particularly during accelerated stability studies, it was observed that the monoclonal antibody MAB001, aggregated faster in sucrose formulation compared to excipient-free formulation at pH 4.8 at 29◦C (13). It was also demonstrated that sucrose hydrolysis and the corresponding formation of glucose lead to formation of glycated antibody which had a higher aggregation tendency. In cases where the formulation has to be liquid and slightly acidic, it is recommended to use trehalose which has remarkable stability against acid hydrolysis. Pfanstiehl’s sucrose is extensively characterized for reducing sugars and such data is available upon request.
For formulators, it is important to be aware of the source of high purity sucrose, which typically comes from beet or cane. The natural source of excipient, purification process, storage conditions and containers could be sources of trace impurities in the sugar excipient and can ultimately affect product quality and stability. In a review, Wang and colleagues summarize the impact of residual impurities and contaminants on protein stability (14). In Brazil, the metal levels in sugar canes could be traced back to the soil quality which had an influence of municipal landfill and a medical waste treatment system (15). In order to ensure product stability and safety, formulators need to be well aware of the excipient’s quality, source and performance in their product. Trace metals are known to promote metal catalyzed oxidation (MCO) of the therapeutic proteins and destabilize them. Such modifications due to MCO can make the proteins potentially aggregation prone (16, 17).
β-GLUCANS & ENDOTOXIN IN HIGH PURITY SUCROSE
β-Glucans are commonly found polysaccharides in sugar-based products. β-Glucans are polysaccharides of D-glucose monomers linked by β-glycosidic bonds. Cellulose, a common polysaccharide comprises of chains of glucose molecules with 1-4 linkage while β-Glucan comprises of chains of glucose molecules with 1-4 linkage with an occasional 1-3 linkage. Several studies describe approaches to control and eliminate β-Glucans from bioprocessing. Sucrose and Cellulose based filters have been identified as common sources of β-Glucan contamination in bioprocessing and in blood products (18-20), these studies also describe approaches to reduce β-Glucan contamination in bioprocesses (19, 20). High amounts of β-Glucans in pharmaceutical products can illicit an immune response and hence it is necessary to control their level in biopharmaceutical products (21-23).
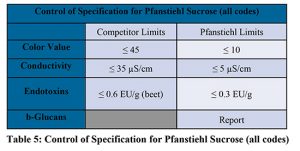
The Limulus amebocyte lysate (LAL) method is widely used to detect and quantitate bacterial endotoxin in pharmaceutical products. (1→3)-ß-D-glucans can interfere and give ‘false positives’ in LAL test (24). Glucan blocker is used to prevent the interference from β-D-Glucans and accurately quantify endotoxin (20). Pfanstiehl quantifies both β-Glucans and endotoxin levels in each lot of sucrose manufactured. Table 4, describes the quantification of endotoxin and β-glucan in multiple lots of cane and beet derived sucrose. Cane derived sucrose has significantly higher amounts of β-Glucans which can interfere with endotoxin quantitation if not sufficiently blocked during endotoxin testing. Additionally, knowing the level of β-glucans is important depending the point in the biopharmaceutical process sucrose is utilized. Pfanstiehl controls specifications for endotoxin and β-glucans as presented in Table 5.
CONCLUSION
In a Quality by Design (QbD) scenario, our approach allows end users to rationally select the well characterized excipients during biopharmaceutical development and develop a control strategy for biologic drug products. Pfanstiehl recommends its customers to discuss their endotoxin and β-glucan control strategy and the methods employed to control these impurities in final product. Pfanstiehl quantifies both β-Glucans and endotoxin levels in Sucrose for biopharmaceutical applications. This approach would help end users rationally develop endotoxin specifications for their product and have a microbial control strategy for manufacturing product of consistent quality.
REFERENCES
- Lee JC, Timasheff SN. The stabilization of proteins by sucrose. J Biol Chem, 1981; 256(14):7193-201.
- Wang, A., Robertson, A. D. and Bolen, D. W. Effects of a naturally occurring compatible osmolyte on the internal dynamics of ribonuclease A. Biochemistry, 1995; 34:15096-104.
- Kendrick, B. S., Chang, B. S., Arakawa, T., Peterson, B., Randolph, T. W., Manning, M. C. and Carpenter, J. F. Preferential exclusion of sucrose from recombinant interleukin-1 receptor antagonist: Role in restricted conformational mobility and compaction of native state. Proc. Natl. Acad. Sci. USA, 1997; 94:11917-11922.
- Liu, Y. and Bolen, D. W. 1995. The peptide backbone plays a dominant role in protein stabilization by naturally occurring osmolytes. Biochemistry 34:12884-12891.
- Sundaramurthi P, Suryanarayanan R. Influence of crystallizing and non-crystallizing cosolutes on trehalose crystallization during freeze-drying. Pharm Res. 2010; 27(11):2384-93.
- Zhou R, Schlam RF, Yin S, Gandhi RB, Adams ML. Investigation of freeze/thaw-related quality attributes of a liquid biopharmaceutical formulation: the role of saccharide excipients. PDA J Pharm Sci Technol. 2012; 66(3):221-35.
- Jena S, Horn J, Suryanarayanan R, Friess W, Aksan A. Effects of Excipient Interactions on the State of the Freeze-Concentrate and Protein Stability. Pharm Res. 2017; 34(2):462-478.
- Jena S, Suryanarayanan R, Aksan A. Mutual Influence of Mannitol and Trehalose on Crystallization Behavior in Frozen Solutions. Pharm Res. 2016; 33(6):1413-25.
- A Platform Approach to Preformulation Development for Antibody Products, Presentation by Tim Kelly PhD, KBI Biopharma Inc.
- Pharmaceutical Development for ADCs. Presentation by Lisa Hardwick and Wendy Saffell-Clemmer. Baxter Biopharma Solutions.
- Yamabe S, Guan W, Sakaki S. Three competitive transition states at the glycosidic bond of sucrose in its acid-catalyzed hydrolysis. J Org Chem. 2013; 78(6):2527–2533.
- Goldberg RN, Tewari YB, Ahluwalia JC. Thermodynamics of the hydrolysis of sucrose. J Biol Chem. 1989; 264(17):9901–9904.
- Banks DD, Hambly DM, Scavezze JL, Siska CC, Stackhouse NL, Gadgil HS. The effect of sucrose hydrolysis on the stability of protein therapeutics during accelerated formulation studies. J Pharm Sci. 2009. 98(12):4501–4510.
- Wang W, Ignatius AA, Thakkar SV. Impact of residual impurities and contaminants on protein stability. J Pharm Sci. 2014; 103(5):1315-30.
- Segura-Munoz SI, da Silva Oliveira A, Nikaido M, Trevilato TM, Bocio A, Takayanagui AM, Domingo JL. Metal levels in sugarcane (Saccharum spp.) samples from an area under the influence of a municipal landfill and a medical waste treatment system in Brazil. Environ Int. 2006; 32(1):52–57.
- Kumar S, Zhou S, Singh SK. Metal ion leachates and the physico-chemical stability of biotherapeutic drug products. Curr Pharm Des. 2014; 20(8):1173-81.
- Mulinacci F, Poirier E, Capelle MA, Gurny R, Arvinte T. Influence of methionine oxidation on the aggregation of recombinant human growth hormone. Eur J Pharm Biopharm. 2013 Sep;85(1):42-52.
- Experimental proof of contamination of blood components by (1→3)-β-D-glucan caused by filtration with cellulose filters in the manufacturing process. J Artif Organs. 2003;6(1):49-54.
- Multipronged approach to managing beta‐glucan contaminants in the downstream process: Control of raw materials and filtration with charge‐modified nylon 6,6 membrane filters. Biotechnol Prog. 2013 May-Jun;29(3):672-80.
- Development of downstream processing to minimize beta‐glucan impurities in GMP‐manufactured therapeutic antibodies. Biotechnol Prog. 2016 Nov;32(6):1494-1502.
- Beta-glucan contamination of pharmaceutical products: How much should we accept? Cancer Immunol Immunother. 2016 Nov;65(11):1289-1301.
- Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J Immunol. 2004, 173 (2): 797-806.
- The effects of β-glucan on human immune and cancer cells. Journal of Hematology & Oncology 2009; 2:25.
- Pharmaceutical Product Impurities: Considering Beta Glucans; American Pharmaceutical Review, August 31, 2013.
Total Page Views: 9657