Credence MedSystems Awarded Gates Foundation Grant to Develop Dual Chamber Drug Delivery Device
Credence MedSystems, an innovator in injectable drug delivery technology for the biopharmaceutical industry, recently announced it has been awarded a grant from the Bill & Melinda Gates Foundation to support the development of Credence’s Dual Chamber Reconstitution Syringe tailored for use in developing nations.
The project will advance development of a safe reconstitution and injection device for administration of drug and vaccine products requiring storage in dried form until the time of use. Drug products must often be stored in dried format to maintain stability and then be reconstituted at the point of administration. Conventional approaches provide significant risk of human error, contamination and needlestick exposure; these risks have been particularly challenging in low-income environments such as those found in the Global Health setting.
“Credence MedSystems is committed to working with the Bill & Melinda Gates Foundation to help all people lead healthy, productive lives,” stated Jeff Shanley, Credence’s Chief Executive Officer. “Enabling the safe, easy and effective delivery of critical therapies in developing nations is an area where Credence can apply its expertise to support the foundation’s mission and help affect meaningful change where it is most needed.”
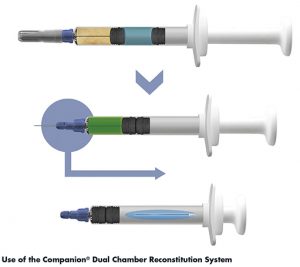
The Companion® Dual Chamber Reconstitution Syringe (C-DCS) is a user-friendly drug delivery system that maintains separation of the drug product from the diluent during storage but allows for simple reconstitution and injection at the point of use. The C-DCS is shipped ready-for-use. The user presses on the thumbpad to transfer liquid from the rear chamber into the front chamber where it mixes with the dried drug product. Upon completion of the injection, an audible and tactile ‘click’ occurs, providing an end-of-dose cue as the needle automatically retracts into the barrel of the syringe. This shields the user from needlestick injury and permanently disables the system from reuse.
A Dual Chamber presentation is a combination product that requires innovation in the device, as well as in drug formulation and fill-finish processing. For this reason, Credence is collaborating on this project with Lyophilization Technology, Inc. (LTI), a CDMO with expertise in lyophilization for healthcare products. Credence and LTI have collaborated on several projects for large and small pharmaceutical manufacturers alike. “It is an extraordinary opportunity to support the venerable ambition of the Gates Foundation in promoting health throughout the world. We are pleased to again collaborate with Credence MedSystems, a pioneering device company developing innovative approaches for the delivery of challenging injectable products,” expressed Ed Trappler, President of LTI.
Upon successfully addressing key technical risks in this phase of the project, it may warrant a more ambitious follow up grant to achieve scale. “The pent-up demand for an easy to use reconstitution and injection device is significant, not just in the developing world but also in more developed regions,” added John Merhige, Credence’s Chief Commercial Officer. “With the prevalence of more-complex biologic molecules that require storage in dried form, and with the administration of healthcare shifting to the home, the Dual Chamber Syringe allows less experienced users to safely and effectively administer their medications.”
About Credence MedSystems, Inc.
Credence MedSystems is an innovator of drug delivery devices that solve unmet market needs. Credence’s philosophy of Innovation Without Change allows our customers to impress and protect their end users while preserving their existing processes, sourcing strategies and preferred primary package components. The Companion® family of syringe systems includes proprietary needle retraction technology, syringe reuse prevention and other critical safety and usability features. The Dual Chamber Reconstitution platform offers single-step mixing and injection for drugs that require reconstitution at the time of delivery. Metered dose systems and other novel devices address the needs of specific therapeutic markets such as ocular therapies and cosmetic applications. For more information, visit www.CredenceMed.com.
Contact: John A. Merhige, jmerhige@credencemed.com
+1-844-263-3797 (844-CMEDSYS)
About Lyophilization Technology, Inc.
LTI is a Contract Development & Manufacturing Organization (CDMO) focused on all aspects of lyophilization for preparation of healthcare products. For more information, visit www.lyotechnology.com.
Total Page Views: 2263