Issue:September 2018
DRUG DELIVERY - Targeted Delivery of Submicron Particle Cancer Chemotherapy: Helping Shift the Immunotherapy Paradigm
INTRODUCTION
Chemotherapy (CT) has been used in the treatment of cancer since the late 1940s. A new class of chemotherapeutic agents called taxanes were introduced in the 1990s and remains one of the key advances in the fight against cancer.
Most CT agents are administered intravenously or orally, with very little drug ultimately delivered to the site of disease to cause a therapeutic effect. Additionally, systemic administration of these cytotoxic agents can lead to system-wide toxicity. As such, dose and frequency of dose must be carefully monitored to cause a therapeutic effect while attempting to limit toxicity. Too often, however, a suboptimal clinical outcome is the result in the form of incomplete tumor response or unacceptable adverse effects.
The subsequent emergence of biotechnology and genomics signaled the future promise of immuno-oncology therapy (IOT). However, the early generations of IOTs have their own drawbacks with only selective effectiveness and significant side effects, and more promising therapies are still years from market approval and widespread use.
Research has shown that CT and IOT in combination are synergistic and may become a mainstay of cancer therapy in the future. Unfortunately, their side effects are additive as well, which is problematic when considering how to optimize their use together.
NanOlogyTM, a clinical-stage pharmaceutical development company, was formed in 2015 to increase the effectiveness and safety of CT through targeted delivery. Based on a proprietary submicron particle production technology, the company has developed stable, uncoated submicron particles of the taxanes, paclitaxel and docetaxel, as investigational drugs, which can be administered directly to the site of disease via injection, instillation, or inhalation.
These particles of pure drug are so unique in terms of size and surface area, they have recently been granted a composition of matter patent. When injected locally, via intratumoral injection for example, research has shown the particles become entrapped at the disease site, releasing drug over several weeks. This high, sustained concentration of drug at the disease site has been shown to significantly decrease or eradicate tumors in preclinical studies. Because of gradual clearance at very low levels, systemic side effects have been shown to be negligible. Moreover, the enhanced tumor kill drives a significant immune response that is being investigated to determine whether the response contributes to tumoricidal activity either alone or in combination with IOT. With five Phase 2 clinical trials underway and more on the horizon, NanOlogy investigational drugs are aiming to help shift the paradigm for IOT.
LIMITATIONS OF SYSTEMIC DELIVERY
Paclitaxel and docetaxel were game changers in the fight against cancer following their market approvals in the 1990s. They are in the taxane class of antimitotic compounds that work by inhibiting cell division leading to cell death. Their impact is greater on rapidly dividing cells, a characteristic of cancer cells but also of some normal cells. The drugs do not discriminate between the two and when administered systemically lead to significant side effects in some patients like bone marrow suppression, nausea, vomiting, and hair loss.
These side effects limit the amount of drug that can be given in a single dose and time until the next dose can be given so a course of CT typically involves administration in 2- to 4-week intervals for up to six cycles. Paclitaxel and docetaxel half-life in the body are both less than 1 day and are normally cleared from the body in only 2 or 3 days. Because of this, drug is at the disease site for a very short time, and multiple cycles are given with the goal of eliminating the tumor over the course of therapy. Too often, tumor burden decreases during treatment, but the disease is not eradicated, or patients cannot tolerate an entire course of therapy. Unfortunately, recurrence or spread of the cancer, or resistance to new treatment, may result.
Nonetheless, paclitaxel and docetaxel are effective cancer killing agents for a wide range of cancers and researchers have long searched for ways to deliver large, sustained amounts of these drugs to the disease site to enhance their efficacy. This has proven to be difficult via systemic administration because no effective ways have been developed to substantially concentrate drug at the disease site for long periods of time. Both drugs are also hydrophobic and solvents and further dilution are required to administer the drugs via intravenous infusion. The solvents themselves are toxic, requiring pretreatment with steroids and antihistamines to prevent allergic reactions. Some success has been achieved in micronizing CT drugs to increase the amount of drug that can be administered, but these require coating agents like albumin or carriers like liposomes to keep them stable. In fact, when researchers refer to “nanoparticles,” they are often referring to the coating or carrier agents upon which drug is placed to enable better oral or systemic bioavailability.
LOCAL DELIVERY OF PURE SUBMICRON DRUG PARTICLES
Pharmaceutical nanoparticle research has mainly focused on increasing the oral or systemic bioavailability of drugs. Making small drug particles is complicated because conventional means, such as milling or microfluidization, impart static energy to the particles causing them to clump together and be difficult to work with. To solve this dilemma, coating agents are used to prevent clumping or carriers are used to complex the drug molecules. Either way, the goal is typically to increase system-wide bioavailability.
The NanOlogy technology turns this around by using local delivery of submicron particles of pure drug using the drug particles as a depot for sustained release to increase local drug concentration and residence time at the disease site. Allowing this is a proprietary submicron particle production technology that uses supercritical fluid carbon dioxide and sonic energy in a nonmechanical process that imparts very little static charge to the particles. As such, the particles remain stable and free-flowing in powder form allowing them to be suspended (not dissolved) in simple diluents at time of use for local delivery in high concentration directly to the disease site.
Unlike intravenous solutions of paclitaxel or docetaxel, which would quickly diffuse out of the tumor if given locally, the NanOlogy submicron particles become entrapped at the disease site, releasing active drug at therapeutic levels for several weeks. Drug clearance from the disease site is gradual, at subtoxic levels, causing negligible systemic adverse effects.
The simple elegance of this approach belies the complexity of production process and the particle specifications including size, geometry, surface area, density, and dissolution that are essential for this effect. Outside the lower specification limit does not allow for sufficient entrapment. Outside the upper limit does not allow for sufficient drug release. The uniqueness of these characteristics is described in a composition of matter patent (US 9,814,685) on the particles that is valid until 2036.
EXTENSIVE PRECLINICAL & CLINICAL DEVELOPMENT PROGRAM
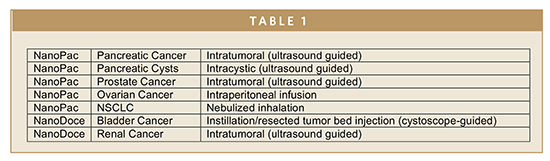
After completing preclinical toxicology and pharmacology studies to support IND approvals, NanOlogy is in clinical evaluation of two investigational drugs including submicron particle paclitaxel suspension identified as NanoPac® and submicron particle docetaxel suspension identified as NanoDoce®. NanoPac is under clinical evaluation for safety and efficacy in Phase 2 trials for the treatment of prostate cancer, pancreatic cancer, mucinous pancreatic cysts, and ovarian cancer with orphan drug designation. The prostate trial will complete in late 2018 and the others within 12 to 18 months. A Phase 2 clinical trial is planned for NanoDoce in bladder cancer in late 2018 and renal cancer in mid-2019. Findings from preclinical pharmacokinetic and pharmacology studies of an inhaled version of NanoPac for non-small cell lung cancer (NSCLC) were presented at ASCO in June. IND-enabling work is underway to allow for a human clinical trial in 2019.
NanOlogy clinical trials are designed to provide human proof of concept to enable delivery optimization and pivotal clinical trials. To date, more than 50 patients have been administered the investigational drugs. NanoPac and NanoDoce are designed for local delivery as described in Table 1.
In the Phase 2 prostate cancer trial, for example, patients undergoing planned prostatectomies have received an intratumoral injection of NanoPac 28 days before surgery. Early data from the open label trial show evidence of tumor reduction, tumor cell death, an immune response, drug in lymph nodes, and no significant drug-related adverse events.
The initial excitement surrounding NanoPac was based on published findings from a Phase 1 clinical trial for ovarian cancer and other peritoneal malignancies. Twenty-one patients, who were suffering from Stage 3 and 4 cancer and had failed all other treatments, received up to 6 cycles of intraperitoneal NanoPac.
The findings showed high and prolonged concentration of drug in the peritoneum via pharmacokinetic analysis with gradual clearance at subtoxic levels with peritoneal concentrations 450-2900 times greater than peak plasma drug concentrations. No bowel obstruction or other significant drug-related adverse events were observed, and five of the seriously ill patients survived at least 400 days after receiving NanoPac. The encouraging results led FDA to grant orphan drug status to NanoPac for ovarian cancer.
Because paclitaxel and docetaxel are FDA approved with more than 20 years of clinical experience, the FDA also allowed its streamlined 505(b)2 regulatory pathway for NanoPac and NanoDoce. It is rare for a company to have a composition of matter patent on products following a 505(b)2 regulatory path.
AN UNEXPECTED IMMUNE RESPONSE
Research has demonstrated that taxanes like paclitaxel and docetaxel can exert a modest immune response as part of their activity, and clinical research is underway using these drugs systemically in combination with IOT to evaluate their value in combination therapy. Unfortunately, systemic CT is fraught with significant adverse effects that add to the side effects of IOT.
Upon histological examination of lung tissue during preclinical lung cancer research, NanOlogy discovered a profound immune response to inhaled NanoPac versus systemic paclitaxel. This enhanced immune response has now also been seen preclinically in bladder, renal, and breast tumors, and clinically in prostate tumors. Immunohistochemistry has demonstrated substantial macrophage and lymphocyte infiltration in and around the tumor site in all cases and tumor eradication in some cases. The scientific rationale for this effect is that large, sustained concentration of drug at the disease site substantially increases tumor kill and local accumulation of dead tumor cell debris. This debris contains tumor specific antigens, which elicit a strong immune response.
NanOlogy is conducting extensive research to confirm these findings because its potential represents a paradigm shift in the treatment of some cancers. That is, local delivery of the NanOlogy investigational drugs by themselves or prior to IOT may jumpstart the immune system and response to therapy without adding to adverse effects associated with systemic CT.
PRODUCING PARTICLES AT COMMERCIAL SCALE
FDA approval of a new drug not only requires proof of safety and efficacy but the ability to reliably manufacture the drug at commercial scale. NanOlogy is related to a company called Phyton Biotech, which is the pioneer in plant cell fermentation (PCF®) for the development and commercial manufacture of plant-derived active pharmaceutical ingredients (APIs) and has become the largest producer of paclitaxel and docetaxel via PCF in the world. With GMP facilities in Hamburg and Vancouver, Phyton sells its APIs in every major geography, including the US, Europe, Japan, and China.
Importantly, because its paclitaxel and docetaxel are uniquely derived via PCF, Phyton is the only company in the world that controls the entire production process of these APIs in house without the negative environmental impact and supply risk associated with harvesting vast yew tree plantations for the API starting material. All other API suppliers in the world rely on harvesting yew tree plantations to source 10-DAB, their common starting material for both paclitaxel and docetaxel.
Engineering plans are in place to transfer the commercial submicron particle production process of NanoPac and NanoDoce to Phyton’s Vancouver facility to take advantage of the facility’s space, infrastructure, and engineering, operational, and quality personnel. Through Phyton, NanOlogy will be able to control API and bulk finished product production in one FDA-inspected facility to facilitate the CMC review and approval of the products.
SUMMARY
With much attention and research investment into IOT, NanOlogy is shaping a new paradigm for cancer treatment by attempting to improve the safety and effectiveness of tried and true CT through local delivery. The standalone potential of NanOlogy investigational drugs is noteworthy. However, the promise of these drugs to help optimize IOT cannot be overstated and could be transformational for the treatment of some cancers by reducing doses and/or cycles of IOT and resulting in better overall clinical outcomes. Because of the streamlined regulatory pathway of the NanOlogy investigational drugs, market approval and access to patients for successful drug candidates could be just a few years away. This paradigm shift has the potential to save more lives, increase patient quality of life, and reduce overall cancer care costs, and provides a pharmaceutical development area with significant investment potential.
AUTHOR’S NOTE
The NanOlogy investigational drugs described in this article are undergoing the preclinical, clinical, and CMC studies required by the US FDA for NDA submission. None of the drugs have been proven to be safe and effective or are approved for commercial distribution in the US or any other jurisdiction.

Marc Iacobucci, is an officer and board member of NanOlogy, Managing Director of DFB, and serves on the executive committee of Phyton Biotech, a company wholly owned and operated by DFB. He is part of a small team responsible for identifying new healthcare investment opportunities and leading them through development to value creation for DFB. Currently, he is primarily involved in advancing a broad clinical program in oncology for DFB affiliate, Nanology, LLC, which was formed in 2015. Prior to his current role, Marc led establishment of Phyton as a commercial operation. Marc has been with DFB since 1993 with leadership roles in business development, marketing, project management, and operations across DFB’s family of companies. Earlier in his career, Marc worked for Procter & Gamble as a market analyst, Merck as a pharmaceutical representative, and as a clinical pharmacist in Ohio and Texas. A graduate of the Ohio State University with a Bachelor of Science degree in Clinical Pharmacy, Marc received his Master of Business Administration from the University of Texas at Austin. For more information about NanOlogy, please visit www.nanology.us.
Total Page Views: 2864


















