Issue:October 2017
GENE & CELL THERAPY - Its Growing Potential to Disrupt Drug Research & Healthcare Delivery
INTRODUCTION
Gene therapy has been emerging for 30 years with the promise of more effectively treating and curing diseases. The basic technology initially used an engineered virus or viral vector to deliver a normal gene to overcome loss of function due to an inherited dysfunctional gene. Vector constructs did not result in sufficient functional gene expression, were not durable, and in some cases, resulted in serious adverse events. With the convergence of an increased understanding of molecular biology catalyzed by the Human Genome Project, new research tools, investments by companies in the industry (American Gene TechnologiesTM, Bluebird Bio, Kite Pharma, Juno Therapeutics and more), a pipeline of promising product candidates are in development and, recently, some commercial products are available in the clinic. Consequently, “modern gene therapy” is maturing and becoming a powerful and practical drug development modality.
Today’s advances in gene and cell therapy are not only about creating new treatment options, but also about discovering durable cures. Curing diseases differentiates modern gene therapy from many treatments available today that primarily help manage diseases. A large portion of today’s healthcare delivery system is devoted to managing chronic diseases, such as cardiovascular disease, diabetes, autoimmune diseases, and cancer. With gene therapy advances, the entire industry will undergo a profound change as a growing number of chronic diseases are cured.
Today’s gene therapy involves delivering genetic constructs that may include regulators of gene expression (either increasing or decreasing gene expression as required), genes encoding natural or non-natural proteins, and functions that modify micro-environmental conditions. The rich and expanding variety of well-understood genetic “tools” allow scientists and researchers to provide solutions to an increasing range of complex diseases, such as infectious diseases and cancers.
At the heart of gene technologies is the precision and power to correct expression of genes that underlie human disease. Ultimately, gene technologies will create a revolution in medicine that may surpass the impact of antibiotics and vaccines. American Gene Technologies (AGT), an emerging genetic medicine company with a proprietary lentiviral platform capable of broad applications, include large and orphan indications, immuno-oncology, and monogenic disorders, as well as other companies in this biotech sector, is at the forefront of a wave of innovation that will bring positive change throughout the healthcare system and relief to millions of patients that currently suffer from untreatable or incurable diseases.
THE GROWTH OF GENE & CELL THERAPY
Although gene therapy was first envisioned in the 70s and attempted in the 80s, the first success was in the early 90s. The early successes in gene therapies, however, often had limited duration or unexpected and dangerous side effects. In the 90s, treating ADA-SCID (sometimes commonly called “bubble boy” disease) worked, but sometimes result in a serious side-effect: although a European experiment to treat ADA-SCID disease result in a cure, it appeared to cause leukemia for 2 out of 10 treated children.
In another gene therapy experiment in the late 90s, a death identified as being caused by a massive immune response to the treatment, temporarily put the brakes on further viral vector research and development in the US.
Nevertheless, gene therapy continued to evolve. Innovations continued in oncology and monogenic disorders. Mechanisms for better control of the delivery of the genetic materials and greater protection from unintended immune responses emerged. Medical research continued to gain insights into the sub-molecular functioning of genetic and biological drivers of diseases that in turn helped scientists develop gene therapies to more narrowly target and more safely treat diseases.
As the industry recovered from early missteps and failures, gene and cell therapy progressed in a manner that will fundamentally change healthcare. GlaxoSmithKline continued development on the ACA-SCID therapeutic removing elements of the viral vector that were thought to be at the root of the leukemia side-effect. Their successful “debugging” of the previous clinical attempt resulted in their year’s approval of StrimvelisTM in Europe. Strimvelis is also expected to be approved in the US. The FDA has granted fast-track designations to a range of gene technology clinical trials, such as Novartis’ CTL019 (tisagenlecleucel) for childhood leukemia and Kite Pharma’s axicabtagene ciloleucel for non-Hodgkin’s lymphoma. The Novartis drug not only received fast-track FDA review, it was unanimously recommended for approval by the FDA advisory committee on July 12, 2017, and is likely to receive FDA approval in September.
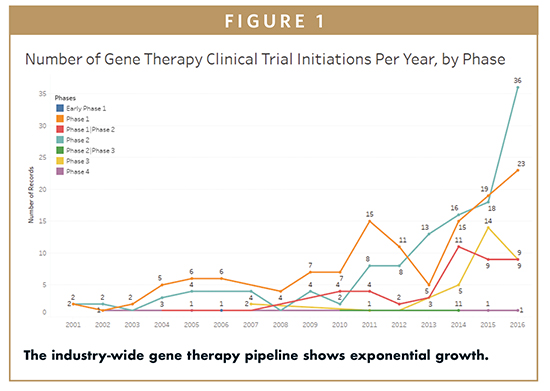
Although the number of approved drugs is still small, the number of gene therapy clinical trials is growing fast. According to clinicaltrials.gov, there were 49, 61, and 78 trials initiated in 2014, 2015, and 2016, respectively; 99 of these trials were in Phases 2 or 3 (see Figure 1). By comparison, prior to 2010, there were never more than 11 such trials initiated. These metrics demonstrate the growing body of evidence that many treatments are fundamentally safe, having moved beyond Phase 1. Many companies are also advancing in the clinic, including Sangamo Therapeutics, AveXis, and Bluebird Bio. Other gene and cell therapy companies, including American Gene Technologies are entering the clinical stage. This breadth of activity underscores the growing momentum of this promising technology.
Why are biotech scientists and key opinion leaders in biotech convinced that modern gene therapy will have a transformative impact on healthcare? The answer can be understood through parallels between computer coding and genetic coding. Human DNA is made up of nucleotides that are composed of four nucleobases (Cytosine, Guanine, Adenine, and Thymine or C,G,A, and T). These combine to form the DNA strands that specify the intricate ways genetic makeup governs how the human body functions. Gene and cell therapy uses this coding, with the growing understanding of the human genome to correct malfunctions (diseases) in the human body in a similar way to “updating” software on a computer. In fact, one could look at the bodies’ coding of our “software” in C,G,A, and T as just a base-four version of computer code that is coded in base-two (0s and 1s). The “software” of the DNA is more complex than computer software, but it has much of the deterministic nature, power, and “rational design” aspects common to the programming of digital computers. The nature of this type of “coding” makes gene technologies a direct connection to the operation of the cell that is both powerful and accurate. The order of the C,G,A, and Ts in genes that make up “commands” to one’s cells largely determine how those cells operate. This is highly similar to the way the order of the 0s and 1s form commands to a computer and determine what the computer executes.
The increasing ability to “hollow out” the malicious viral code inside viruses that have been infecting human bodies, now allows us the repurposing of a viruses’ innate ability to deliver benevolent (update) code that improves the reliability of the programing. In fact, these new “updates” have the potential to eradicate viruses the way that updates on computers can repair viruses on a PC, Mac, or other computer device. Now there is have a swiftly growing ability to deliver DNA “updates” to specific cells in the body that can target disease much more accurately than traditional pharmaceuticals, and that act on those cells in highly specific ways. The result is a new class of drugs that powerfully change the root drivers of disease exactly where it resides, and highly limits treatment (and side effects) to non-diseased cells.
The confluence of the increased ability to deliver new “code” to cells, coupled with medical researchers’ in depth understanding of the intricate workings of genes that determine cellular functions is presenting an opportunity to create a huge number of therapeutics that can substantially grow the ability to effectively (and efficiently) treat and cure disease.
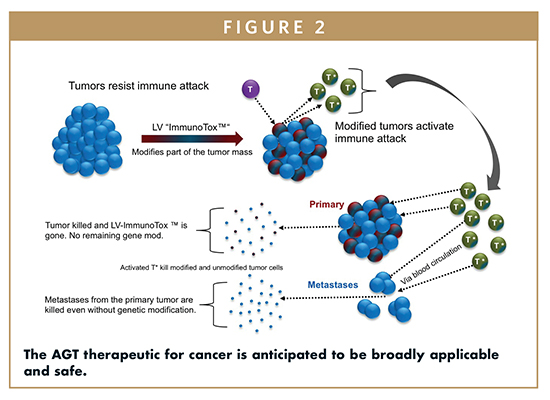
The clinical application of this technology can span a broad set of serious diseases. AGT believes that immune-oncology can be significantly improved and expanded into solid tumors (which include most terminal cancers). AGT, has taken an alternative and innovative approach that “reprograms” cancer cells by lowering their defenses and secretory factors. This allows specialized cancer-surveillance T cells to greatly increase their activity against the tumor mass (up to 600 times of normal). AGT’s therapeutics use the power of immuno-oncology without modifying the immune system itself. This is a critical innovation that can reduce the possibility of long-term side effects that have been a challenge to moving CAR-T forward into a wider variety of cancers.
Monogenic diseases are also an obvious target for gene therapy cures. They tend to be the result of single gene mutations or dysfunctions that can lend themselves to viral vector-delivered gene replacement. For example, AGT has a strategy to “reprogram” a set of cells in phenylketonuria (PKU) patients to permanently supply sufficient quantities of phenylalanine hydroxylate to mitigate the disease. This could result in a lifetime cure of this debilitating disease by restoring a critical biological pathway that would prevent the toxic buildup of phenylalanine in the blood of PKU patients while providing essential tyrosine downstream to maintain healthy development of nerves in the brain.
Another gene therapy class of targets is infectious diseases. Serious viral infections, including Hepatitis B and C, Tuberculosis, and HIV can be inhibited or blocked through a range of gene therapy approaches. AGT’s first clinical trial will be an HIV functional cure in the first quarter of 2018. AGT’s therapeutic strategy uses gene modulation both to reduce the levels of CCR5 (which can prevent cells from being penetrated by HIV thus preventing HIV from “planting” itself in healthy cells) and shut down the HIV gene expression in infected cells (thus restoring normal function to the infected cells and preventing the virus from producing new virions that attempt to infect neighboring cells). AGT’s therapeutic lentivirus and cell therapy treatment is expected to restore natural immunity to HIV in HIV-infected persons; mitigating the effects of the disease and even preventing reinfection over the remainder of an individual’s lifetime.
THE TRANSFORMATION HAS BEGUN
What has begun as a trickle of highly effective gene and cell therapeutics will soon become a steady stream. Eventually, a tidal-wave of cures and treatments will benefit patients and profoundly change healthcare outcomes forever. CAR-T immunotherapy and monogenic gene therapies using a range of viral vectors are at the forefront of this growing tide. There are many other gene technology companies lining up to lead, follow, or support this field.
The coming revolution in disease therapies will not only change health treatments and outcomes, it will fundamentally change care delivery. Today’s medical products and treatments are created and delivered around an economic model that substantially depends on chronic or sustained treatment of non-acute illness. Many core components of the healthcare system are on the fault line of gene therapy disruption, including non-curative blockbuster drugs based on older drug technologies; devices that diagnose, monitor, and manage illness; care delivery; medical staffing; and standards of care that specify periodic visits, tests, and the modifications of chronic care treatments. Sustained, long-term cures will greatly reduce or eliminate the need for many of these services and the products used to manage the diseases.
CONCLUSION
Across the industry, biotech companies are making significant progress in driving the shift from disease management to cures. This transition will result in the profound disruption of the healthcare industry as many current treatments are replaced by more effective genetic treatments and even cures. As gene and cell therapy become established therapeutic modalities, a portion of pharma companies will adapt to these changes by incorporating the technologies into their own therapeutic discovery efforts, as well as aggressively in-licensing new gene therapeutics at increasingly earlier development stages.
As the pace of gene and cell therapies accelerates over this next decade, potential cures for chronic diseases (diabetes, RA, hypertension, Alzheimer’s, Parkinson’s etc.), cancer cures, and autosomal (inherited) defect cures will result. The efficacy of new therapeutics may move as much as $500 billion (per year in revenue) from traditional pharmaceuticals to gene technologies. Overall, treatment costs are likely to go down, not only because the cost and payment models will be different, but also because cures for diseases will be less costly overall than the long-term expense of traditional chronic care and palliative treatment of serious human disorders.
While pharmaceutical and healthcare delivery companies retool themselves to adapt to (and even survive) the sea-change in medicine, the patient and the public will benefit with increased solutions to traditionally evasive health disorders; solutions that raise the efficacy bar while lowering overall costs – providing a significantly more economically efficient healthcare system.
To view this issue and all back issues online, please visit www.drug-dev.com.

Jeff Galvin is the CEO and Co-Founder of American Gene TechnologiesTM (AGT). He earned his BA in Economics from Harvard in 1981 and has more than 30 years of business and entrepreneurial experience, including founder or executive positions at a variety of Silicon Valley startups. His meeting of AGT’s Co-Founder Dr. Roscoe Brady in 2006 inspired his belief in gene therapy to advance needed cures to the clinic. For more information: info@americangene.com, www.americangene.com, or (800) 888-9480.
Total Page Views: 4653









