Issue:November/December 2014
IVD TRENDS - In Vitro Diagnostics Players Go Global as US & Europe Markets Slow Down
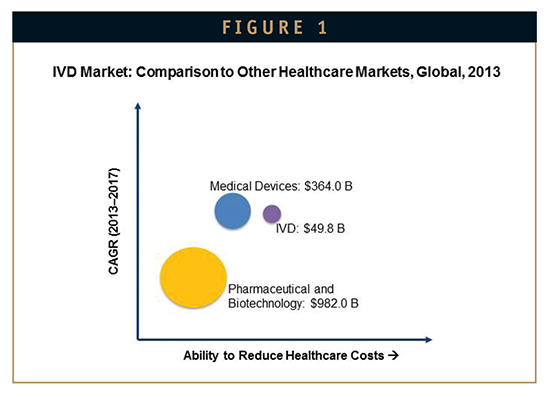
Despite economic and industry challenges, the global in vitro diagnostics (IVD) market is growing robustly – at double the rate of the global pharmaceutical industry (Figure 1). The market will remain buoyant as the recent success of cost-saving companion diagnostics tests and personalized medicine is driving the uptake of various IVD tests and opening up the opportunity to expand test menus. While the US and European markets remain important, their slowdown is demanding an alignment with the global market. As a result, the Asia-Pacific region is becoming a lucrative destination for IVD manufacturers.
New analysis from Frost & Sullivan’s Analysis of the Global In Vitro Diagnostics Market finds the market earned $49.8 billion in revenue in 2013 and estimates this to reach $66.1 billion in 2017. The research covers immunochemistry, self-monitoring blood glucose (SMBG), point-of-care testing (POCT), molecular diagnostics, hematology, clinical microbiology, hemostasis, and tissue diagnostics.
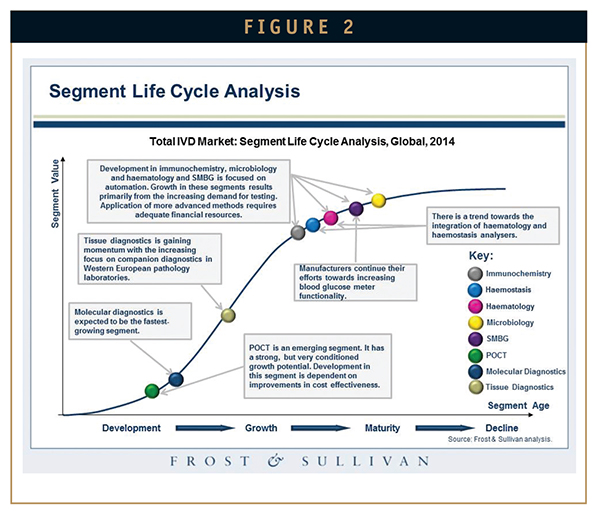
The broad application potential, combined with downward pricing trends enabled by microfluidics integration, is fuelling the long-term growth of the point of care testing (POCT) segment. In spite of market uncertainties, the hematology segment too is gaining traction through the launch of new products, support of an existing installed base, and needs of emerging markets.
In addition to these segments, the molecular diagnostics space holds promise due to continued demand from the developed US and European markets. However, low public health reimbursement for SMBG products in the US and Europe is adversely affecting the development of this segment. IVD companies are pursuing emerging markets to compensate for the drop in market pace in developed nations. With pricing pressures and intensifying competition pervading emerging markets, IVD companies need to operate strategically in these territories (Figure 2).
Further, strained laboratory budgets, workforce shortages, and fewer visits to the doctor by people who have lost their employer-sponsored health insurance are hindering the sale of IVD tests globally. Insufficient connectivity in healthcare facilities is limiting the ability to provide diagnostic testing, adding to market woes.
Nevertheless, as diagnostic testing moves toward process simplicity and decentralization, demand will rise. Not only will this encourage entry into the molecular diagnostic market through acquisitions but it will also increase penetration of biomarkers that can be tested at a point of care level.
Market participants need to employ a diverse set of strategies rather than rely on comprehensive product portfolios to expand their businesses. A mix of the following strategies are expected to be implemented: investing in next-generation sequencing, strengthening product portfolio in a specific area, venturing into emerging markets by establishing partnerships with local companies, acquiring a clinical laboratory improvement amendments (CLIA)-certified laboratory to rapidly commercialize new diagnostic tests, outlicensing of proprietary technology platforms or collaborations with major research institutes, integrating big data into product development and increasing connectivity of devices, and offering pared down personalized machines to improve access and clinical development.
To view this issue and all back issues online, please visit www.drug-dev.com.

Divyaa Ravishankar is a Senior Industry Analyst for Frost & Sullivan’s Life Sciences practice. She has diverse expertise within healthcare IT and life sciences with a focus on in vitro diagnostics. Her expertise constitutes of laboratory research and management consulting. Ms. Ravishankar earned her MS (Hons.) in Biological Sciences from Birla Institute of Technology and Science, Pilani, India. For more information on Frost & Sullivan’s global Life Sciences practice and offerings, please email jennifer.carson@frost.com or visit www.frost.com.
Total Page Views: 7031