Issue:March 2013
DRUG DEVICES - Intranasal Saline: Can a Spray Per Day Keep the Doctor Away?
The use of intranasal saline to wash the nasal cavity, its use as nasal decongestant, or for moistening has a long tradition going back to the Ayurvedic medicine in India. Here, the roots of the famous Neti pot can be found: an item used to pour between 250 and 500 ml of warmed saline trough the nasal cavity to wash away thickened mucus. This procedure is a real “nasal lavage” or nasal wash, which may reach even the sinus cavities. On the other side – speaking in terms of volume – we find nasal droppers and spray pumps, which deliver roughly 50 microliters per drop or 100 to 140 microliters per stroke, used to moisten the nasal cavity. This moistening will relieve nasal congestion when suffering a cold and is useful to keep the nasal mucosa wet in a dry environment (eg, air conditioned offices). In this overview, the benefits of using intranasal saline, and a range of different devices on the market allowing administration into the nose, will be discussed.
BACKGROUND ON NASAL SALINE
Under resting conditions, an adult breathes about 10,000 L of air per day, in and out. Humans are designed by nature to be “nose breathers.” So the air should go its way through the nasal cavity, where it is warmed and moistened close to 100% relative humidity. Dust particles and microbes are trapped there and bound by the mucus layer. This mucus layer is continuously renewed, and mucociliary clearance mechanisms bring it into the throat portion, where it is swallowed. This is a very effective mechanism and prevents dust and microbes from reaching the lower respiratory tract. It is also a well balanced system, and everyone will recognize when it is disturbed due to excess mucus production, swollen mucosa, nasal polyps, or foreign particles. So when you consciously realize you are breathing (eg, due to a blocked nose), it is most likely because something is going wrong, and you should see a doctor. But how can saline help here?
The activity of nasal saline within the nasal cavity is mainly physical action. The saline will help to remove the excess mucus and improve mucociliary clearance. This will support nasal breathing in acute upper respiratory tract infections, including the common cold and rhinosinusitis.1 In addition to these cleaning effects, there may be other benefits coming from trace elements in sea water or intentionally added minerals to create so-called “enriched waters.” According to some clinical studies, prophylactic use of moistening sprays can reduce the number of viral infections when taken regularly.2 The mechanisms behind this observation are not clear, but regular saline sprays may prevent drying out of the mucus layer and help to maintain the aforementioned natural defense mechanisms. Also, the action of trace minerals may play a role here. Nasal saline reduces swelling of the nasal mucosa and is therefore recommended as a nasal decongestant in response to an infection or allergy (hay fever). Lower volumes of saline administered as drops (often used for toddlers) or sprays will dilute the highly viscous mucus, which may be enough to improve or to re-start the mucociliary clearance mechanisms. Higher volumes of saline will actually wash away the largest part of mucus and debris from the nasal cavity. This is a recommended procedure for people suffering from chronic rhinosinusitis.3

There are different saline solutions available that differ in the concentration of the main ingredient sodium chloride. A 0.9% solution is called physiological saline because the osmotic pressure is similar to the human blood (~300 mOsm/L). Higher concentrations up to 2.5% to 3% (~ 1 Osm/L) are called hypertonic and are preferred for decongestant purposes due to their osmotic effect. However, too high of a concentration could irritate the nasal mucosa. Sea water offers an interesting alternative due to a wide range of bioavailable elements. The desired osmolarity can be adjusted by means of dilution, as sea water often has a high salinity. Another well-known product for nasal rinsing is the so called Ringer solution: an isotonic salt solution with added bicarbonates or lactate as pH buffer. Clinical studies on the influence of different salt concentrations on symptom relief are not very conclusive. So it is the choice of users to decide on their osmotic preferences.
NETI POT & LARGE-VOLUME SQUEEZE BOTTLES
There are many ways to deliver a saline solution into the nose. The Neti pot may be one of the oldest devices to rinse the nasal cavity. The pot is filled with warmed saline and then poured into one nostril, finding its way through the nasal cavity and allowing it to drain out of the other nostril. Due to the comparable high volume, mucus and debris are washed out, and even the sinus cavities may be reached. It sounds simple, but the procedure needs some exercise and may be not everyone’s preference. It is recommended by physicians as an efficient way to relieve symptoms of rhinosinusitis. On the other hand, some clinical studies suggest that a too intensive use of the Neti pot is linked to higher rates of bacterial and viral infections. Ready-to-use saline preparations appear to be expensive in relation to volume-consuming Neti pot. Prepacked concentrates or self-prepared saline solutions offer a cost-effective alternative but bear the extremely rare risk of microbial infections if the hygienic standards are neglected.
Large squeeze bottles used to instill high quantities of saline solution, again as a constant rinse, are using a “low positive pressure” and can be considered quite similar to the Neti pot.
NASAL DROPPERS FOR CHILDREN
Cleaning or blowing toddlers and young children’s stuffy noses are highly facilitated by the use of nasal saline, reducing complications due to infections of the upper respiratory tract. Some parents and physicians are reluctant to use active drugs, such as xylometazolin in patients of that age group. Saline drops or sprays for these little noses are a great help to restore normal nasal breathing. Unpreserved saline mono-dose droppers manufactured with blow-fill-seal technology are widely used. Also, squeeze bottles or bottles supplied with pipettes are common. From a packaging point of view, this simple technology is relatively easy to use but does not meet the highest hygienic standards because mucus may be sucked back unintentionally. For this reason, such multi-dose drops are often preserved (eg, containing benzalkonium chloride, BAC).
NASAL SPRAYS FOR MOISTENING
There is a wide range of nasal saline sprays commercially available on the market. Their rather small packaging size (20 to 30 ml) is also highly convenient for on-the-go use. The delivered dose varies typically between 50 to 140 microliters per actuation, and the product is physiological or hypertonic saline, sometimes enriched with minerals or herbal extracts for specific use. Preserved and nonpreserved products can be found on the market. In the first case, the saline solution contains benzalkonium chloride to prevent bacterial contamination of the bottle content. Metered pumps used for preserved formulations are well known and proven. Such spray pumps have an actuator with an open swirling chamber, so there is some remaining risk of sucking back small amounts of nasal mucus. So preservatives offer here a safety margin in terms of product purity. It is a mistaken idea that the preservative in the saline or the saline itself will knock out viruses and bacteria in the nose. A potential issue for the user, however, is the high incidence of local side effects attributed to preservatives. The discussion is controversial, and published data is not always consistent. It seems to be clear that short-term use of preparations containing preservatives at low concentrations is well tolerated, but preservatives can cause serious inflammatory effects with long-term use. The responses may include chemical irritation, hyperreactivity, and true allergies.4 The German Authorities (BfArM) addressed the use of BAC for nasal sprays in 2003, which pushed preservative-free systems for this administration route.5 Today, PFS pumps are one of the most sophisticated technologies for moisturizing nasal sprays.
A significant risk of contamination obviously comes from the orifice of the spray system, as it is potentially in contact with the infected nasal mucus. Some marketed systems use the oligodynamic activity of a silver wire in the open tip of the actuator.6 These components release silver ions into the saline, which remain inside the actuator in between the dosing intervals. The system is able to keep concentrations of microorganisms at a low level between long dosing intervals, even when the tip is immersed into bacterialcontaminated fluid.7 Silver ions are widely used for their antiseptic properties, and even when used for wound dressings, it is safe with no adverse effects attributed to this treatment.
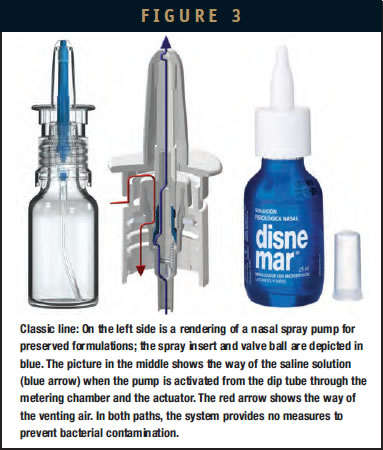
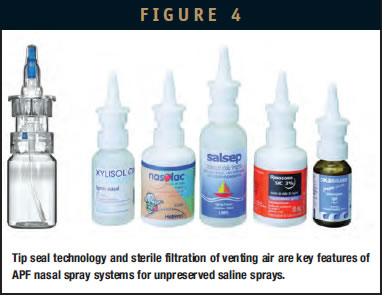
The most recent preservative-free systems follow a purely mechanical approach. One technical solution to prevent contamination via the orifice is referred to as “tip seal technology.” A spring-loaded valve is located directly below the opening of the tip orifice and does not allow any sucking back of liquid and consequently keeps microbes from migrating into the system. Under resting conditions, the orifice is “sealed.” The tip seal keeps the system closed until a defined pressure (for nasal sprays, it is more than 3 bar) is reached by actuating the system. Then the system will open, and the saline solution is forced through the orifice with a higher pressure than needed to open the valve. When the pressure drops at the end of the actuation, the tip seal will immediately close the orifice with an outward movement, preventing any nasal mucus back flow into the device.
Unless equilibrated, throughout the use of product, the pressure within the packaging is decreasing gradually. To avoid product contamination via the venting air, different technical solutions are used. One solution consists of sterile filtration of the venting air using build-in filters or filter gaskets. Also, so-called depressed systems are used. These pumps are designed in such a way that the entire system is air-tight, resulting in an up to 300 mbar underpressure building up during use in the bottle.
Today multi-dose preservative-free devices, capitalizing on advanced and proven technologies, offer a real alternative to preserved formulations. Independent of which technology is used, all will work best in an upright position when the dip tube is submersed into the saline solution. This is no problem for self-treatment. However, caregivers will face problems when administering the product to lying down patients or to children, babies, or toddlers. To overcome this issue, specially designed pumps or containers (eg, systems with collapsing bags) are used to enable spraying in any position (so-called 360° pump systems). Discussing such technologies cannot end without considering a system spraying in any angle: the bag-on-valve (BOV).
BOV
The BOV system consists of a continuous aerosol valve connected to a welded bag. The bag is placed in a can in most cases made of aluminum. A mounting cup will close the bottle and hold the valve and actuator. Prior to product filling, the container is pressurized using an under-the-cup gassing station, providing compressed air or nitrogen into the space between bag and can. Then the product is filled via the valve into the bag. When pressing the nasal actuator, the saline is dispensed by the pressure the compressed gas provides by squeezing the bag. It is important to understand that the compressed air is at no time in contact with the product dispensed. Furthermore, the system does not require any pumping, and its continuous valve allows determining the amount of product needed for each actuation. Depending on the nasal actuator’s type, spraying will result in a gentle mist or jets of different flow rates for specific cleaning/rinsing purposes. For nasal saline, bag sizes are usually between 30 and 200 ml. The system is a real 360° device: it will work in any position independent from the level of remaining content. In addition, BOV technology is preservative-free technology (PFS) and environment friendly due to the use of compressed air rather than any harmful propellant. The same applies for the aluminum aerosol container that can easily be recycled.

Similar concepts exist on the market using various material and bag designs as well as low-pressure systems. For example, the low pressurized POWER ASSEMBLY (ATMOS) system using the power of a rubber sleeve around a specially designed flexible bag. Another system (eg, NOATECH® flexpack) uses a flexible bag made of silicone. When filled with product, the tension force of the silicone bag is sufficient to dispense the content. Various accessories will ensure the desired intensity of the spray. For these systems, plastic bottles are generally used as secondary packaging as not exposed to pressure. Filling process of such devices generally requires the use of a dosage cylinder unit, quite similar to BOV systems.
SUMMARY
Today, nasal saline is a well-established market that continues to grow with sustainable rates. Ways of use, prevention, and treatment have convinced an increasing number not only of consumers, but also experts in the pharmaceutical field, such as ENT specialists and scientists. The choice of the right saline solution, ranging from physiological saline to sea water and even enriched water is in the hands of the users, who have to find out what is best for their noses. The same applies for packaging technologies offering a wide range of dispensing systems dedicated to nasal saline. Considering the title of this article, claiming that “a saline spray a day keeps the doctor away” is not that unwise. Among various products, the market has fully adopted nasal saline as an adequate and effective product for itself, but also as an adjunctive therapy in combination with medicated products. Those who can see further than the tip of their nose, will recognize the high potential of nasal saline.
REFERENCES
1. Kassel JC, King D, Spurling GKP. Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database of Systematic Reviews 2010, Issue 3. Art. No.: CD006821. DOI: 10.1002/14651858.CD006821.pub2.
2. Tano L, Tano K. A daily nasal spray with saline prevents symptoms of rhinitis. Acta Otolaryngol. 2004;124(9):1059-1062.
3. Hong J, Bielory L. Allergy to ophthalmic preservatives. Curr Opin Allergy Clin Immunol. 2009;9(5):447-453. 4. Bescheid des Bundesinstitut für Arzneimittel und Medizinproduke für benzalkoniumchlorid-haltige Arzneimittel zur Anwendung in der Nase, A 37489/38186/03 Bonn, Dezember 2003.
5. Hauptman G, Ryan MW. The effect of saline solutions on nasal patency and mucociliary clearance in rhinosinusitis patients. Otolaryngol Head Neck Surg. 2007;137(5):815-821.
6. Groß D. The COMOD-System – a preservative free drug therapy against glaucoma. 321-328, in Orgül/Flammer (Editors): Pharmacotherapie in glaucoma, Bern 2000.
7. Bagel S, Wiedemann B. Extension of in-use stability of preservativefree nasalia. Eur J Pharmaceut Biopharmaceut. 2004;(57):353-358.

Following the study of veterinary medicine and the successful completion of his thesis at the University of Leipzig, Dr. Degenhard Marx joined the Arzneimittelwerke Dresden/Asta Medica co-operate research in 1992. In 2001, he took over a senior research position at Altana Pharma/Nycomed in Constance, Germany. During this time in the pharmaceutical industry, he collected ample experiences in the drug development of anti-inflammatory and cardiovascular drugs. In 2008, he became Business Development Manager at Ing. E. Pfeiffer, Pharma Division, which became Aptar Pharma in 2010. Currently, he is Director of Scientific Affairs within the Aptar Pharma Consumer Health Care Division.

Georges Bouille is Vice President of Business Development – Consumer Health Care Division – Aptar Pharma. In the frame of Aptar Global Market Development organization, Mr. Bouille is in charge of both Nasal Saline and Wound Care application fields and strategy, capitalizing on over 20 years of experience in dispensing and spray technology. From 1991 and onward, he challenged and succeeded the introduction of the new EP Spray Systems’ dispensing technology – Bag-on-Valve (BOV). Today, he is known as one of the leading WW specialist in that field. After graduating in Economics in 1987 in Neuchâtel (Switzerland), he started as a business consultant before joining an internationally renowned company in the packaging industry for aerosols. Mr. Bouille is based in Switzerland and has an excellent technical aptitude inherited from a Swiss watch manufacturing origin, with a unique passion for marketing and innovation.
Total Page Views: 11083














