Issue:June 2014
INJECTABLE MICROEMULSIONS - Prolonged-Release Injectable Microemulsions: Opportunities for Pain Treatment
ABSTRACT
Recurrent pain in several conditions demands repeated oral or parenteral dosing. However, available therapies do not provide palliative care without limitations. For example, oral treatment efficacy demonstrates significant dose-response variability leading to non-response in several cases. Injectable formulations have their own challenges. Novel formulations that can provide prolonged release promise to bring significant benefits for both-modes of drug delivery as well as treatment of pain. However, available technologies do not support development of such formulations due to challenges associated with complex development and manufacturing procedures, limited excipient and process options, as well as high cost for the finished product. Microemulsions with their unique features can provide a viable alternative to develop such formulations.
INTRODUCTION
The International Association for the study of pain (IASP) defines pain as an unpleasant sensory or emotional experience associated with actual or potential damage. While pain has been classified into acute or chronic pain (lasting for more than 12 weeks), both may be equally debilitating in nature, especially when they persist beyond 24 hours. As per the American Academy of Pain Medicine, 26% of Americans aged 20 years or older, and 30% of those between 45 to 64 years, reported pain that lasted for more than 24 hours.1 Hence, while chronic pain patients need long-term medication, even acute pain patients often need medication for more than 1 day. Various approved analgesic medications are used based on the type of pain, as well as other factors, which include severity of pain, pathological condition associated with pain (eg, malignant or non-malignant pain, postoperative pain, headache), and the age of the patient (eg, patients older or younger than 65 years). For example, the WHO 3-step analgesic ladder for treating cancer pain suggests initiating treatment with oral non-opioids administered every 3 to 6 hours (ie, by the clock rather than on demand).2 However, opioids are the first-line treatment for acute postoperative pain, and are very often used in the form of patient-controlled analgesia (PCA).3
These different requirements have led to the development of a variety of formulations other than conventional immediate-release solid oral and injectable formulations for various analgesic agents, eg, orally disintegrating tablets, transmucosal lozenges, extended-release tablets/capsules, prefilled syringes, and so on. Each of these specialized formulations has unique features in terms of dosing convenience, speed and extent of absorption, onset, and duration of action. However, there are still significant unmet needs as evident by the continuous effort to develop better products. For example, a single dose of Exparel®, a liposomal formulation of bupivacaine, was approved in 2011. The formulation administered locally into the soft tissues at the surgical site provides effective analgesia for up to 72 hours in contrast to the non-liposomal formulations, which need to be injected every 3 hours. However, the employment of the complex technology platforms makes new product development and manufacturing challenging, or adds to the product cost significantly, or fails to meet desired safety/efficacy norms. In the case of Exparel, its cost may be viewed as significantly high ($285 per 20-ml vial) compared to non-liposomal formulations ($10 to $15 per 20-ml vial). Depodur®, an intramuscular (IM) injectable formulation of extended-release liposomal morphine sulphate, was withdrawn due to incidence of adverse events and higher cost ($327 to $491 per dose compared to morphine sulphate injection at $1 per dose). Thus, while new improvised formulations are required for delivering effective pain treatment, it is also important to use a technology that is technically as well as commercially viable.
Microemulsions, comprising an oil phase, aqueous phase, and surfactant/cosurfactant phase, are spontaneously forming isotropic monophasic systems. Their unique features combined with the ability to maneuver their functional performance makes them a promising technology to design prolonged- release injectable formulations.4 While injectable microemulsions have been evaluated primarily for the intravenous (IV) route, the subcutaneous (SC) and IM routes will enable prolonging in vivo drug release beyond 24 hours, and potentially for multiple days similar to Depodur or Exparel.5-7
INJECTABLE THERAPIES FOR MODERATE-TO-SEVERE PAIN: PREVAILING TREATMENT MODALITIES & CHALLENGES
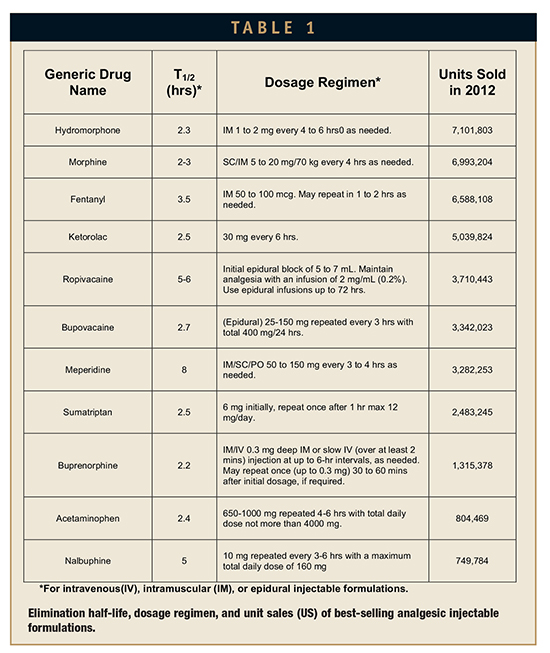
The majority of moderate-to-severe pain conditions are treated with non-steroidal anti-inflammatory agents (NSAIDs), opioid agents, or anaesthetic agents. Use of injectable formulations is often preferred in severe pain conditions due to fast onset and a more consistent response compared to oral formulations.3 Table 1 shows the top injectable analgesic drugs as per the total units sold in the year 2012. The table also shows elimination half-life and dosage regimen for the drugs in respective formulations. As can be seen in the table, most of these drugs have elimination half-lives in the ranges of 2 to 3 hours, indicating their analgesic effect would vanish rapidly after injection. This short half-life necessitates repeated dosing (which may be as much as 3 to 4 times a day as evident by the dosing regimens shown in the table) or infusion to realize adequate analgesia. Injectable drugs are often considered as first-line treatment for moderate-to-severe pain conditions.3 However, the burden associated with their frequent administration, patients’ needle-phobia, clinical visits, need of getting treatment in in-patient set-up, and consequences of missed dose often discourage patients’ preference for injectable formulations. Fosnocht et al have reported that even patients suffering with severe pain prefer oral medication over injectables.8 However, the proportion of patients opting for injectable formulations increases significantly as the severity of pain increases, indicating that patients are aware that injectable formulations would provide faster pain relief compared to oral formulations. Todd et al reported that in an emergency room setting, the majority of long bone fracture patients suffering with moderate-to-severe pain preferred the parenteral route over oral medication.9 While patients prefer faster onset of analgesia, they also prefer treatment that results into lower adverse events and hence are often willing to trade off pain relief for less severe side effects.10 Conventional injectable formulations as those approved result in dose dumping, resulting into higher initial exposure and higher peak-trough fluctuation due to faster elimination. This increases the adverse events associated with injectable formulations. For example, 42% of subjects receiving Imitrex® injection (sumatriptan 6 mg) reported atypical sensation against 6% of those treated with oral Imitrex tablet (sumatriptan 100 mg). Different classes of analgesics have their own set of problems that strongly influences their choice which, in some cases, results in under-treatment, also known as oligoanalgesia. For example, opoiophobia (due to concerns related to opioid abuse as well as severe adverse events like respiratory depression) drives physicians to be over-selective while prescribing opioids, while concerns related to adverse effects, like bleeding from NSAIDs, limits their duration of use.11 Both of these events result in oligoanalgesia.
Treatment of patients discharged from emergency rooms is also important. Patients often report similar pain intensity (ie, moderate to severe) post-discharge as that during their hospital stay, and a significant proportion of them continue the same medication they were administered in the hospital.12 However, in many cases, patients fail to take their medications, resulting in oiligoanalgesia.
Analysis of these facts vis-à-vis pharmacokinetic (PK) and pharmacodynamics (PD) attributes of products highlight the following key points:
- Patient suffering with moderate-tosevere pain needs multiple doses for more than a day. For example, postsurgical patients may need medication up to 96 hours for adequate pain relief. While oral medications are preferred due to convenience, variable response resulting in unsatisfactory pain relief leads to discontinuation and hospital visits in several cases.
- Patients are aware that injectable formulations provide rapid pain relief. While patients prefer oral medications in general, more of them tend to use injectable formulations as the pain intensity increases. However, concerns related to the injectable route (as previously highlighted) would still limit the actual number of use.
Injectable formulations that release the drug for more than 24 hours, more preferably for 72 to 96 hours, would result in reduction of dosing frequency, higher use of injectables, and better pain relief. Recent efforts to develop products like Exparel and Depodur that serve similar objectives is the evidence of the acute need of such formulations.
POTENTIAL TO DEVELOP PROLONGED-RELEASE INJECTABLE FORMULATIONS
Microemulsions for Parenteral Delivery: Advantages
Microemulsions have several unique features. Microemulsions are thermodynamically stable systems. In simple terms, dispersed phase in microemulsion does not undergo coalescence or flocculation. This stability is achieved due to emulsifier film (comprising surfactant and cosurfactant) as well as small globule size that result in very high Brownian motion, which neutralizes the effect of gravitational force.13 The emulsifier film is disordered and fluid due to voids created by cosurfactant molecules penetrating surfactant film.14,15 This film has higher surface pressure compared to the surface tension between the immiscible phases in absence of surfactant.13,16 Thus, mixing of the immiscible phases with surfactant/cosurfactant mixture, and consequent net negative interfacial tension created at the interphase leads to a spontaneous breakdown of dispersed phase (ie, phase with smaller volume fraction) into small droplets, which remain stable for its entire shelf-life. Propofol microemulsion product’s mean globule size of 69.3 nm (t=0) remained stable up to 6 months at (20°C/65%RH) with the mean globule size 66.4 nm at the end of 6 months.17
The technically simple preparation method of microemulsions is a major advantage. Emulsions or nanoemulsions often need multiple cycles of mixing, homogenization, or sonication to reduce globule size to the desired range, achieve uniform size distribution, and ensure absence of larger droplets so as to meet pharmacopoeial requirement (USP chapter <729>), which specifies that the mean globule size for injectable emulsions should be below 500 nm, while percentage of globules with size >5 microns should not be more than 0.05% of dispersed phase. Such processes are strongly influenced by the composition of the formulation.18 In the case of microemulsions, the spontaneous (or a little external energy mediated) breakdown of inner phase to globules, which are usually below 140 nm with narrow size distribution, significantly simplifies the manufacturing procedure.
Filter-sterilizability of microemulsions is another major advantage, as other technologies, including liposomes, often need complex and costly aseptic manufacturing conditions due to large globule/particle size or higher viscosity.19
Prolonged-Release Microemulsions: Factors Influencing Drug Release
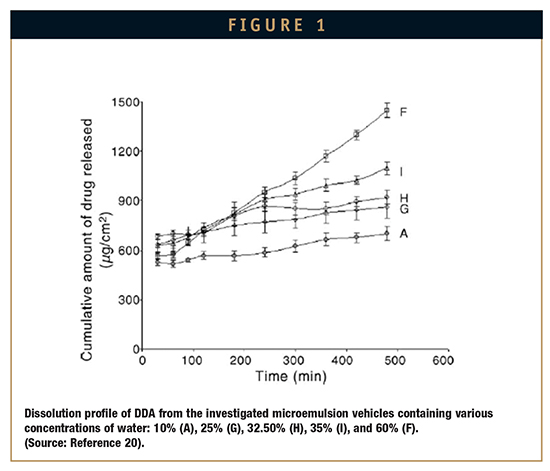
The typical structure of microemulsions, comprising dispersed droplets surrounded by emulsifier film in a continuous phase provides a significant barrier to drug dissolved in inner phase against its rapid diffusion to external phase. The rate of drug release from microemulsions is influenced by the factors related to the drug molecule (eg, logP, pKa, solubility, etc), type of microemulsion (o/w, w/o, or bicontinous), and proportion of the constituents. Thus, a careful selection of constituents (ie, oil, surfactant, cosurfactant), and formulation characteristics (eg, pH) vis-à-vis drug characteristics would help to modulate drug release for microemulsions. Djordjevic et al prepared w/o microemulsion containing an amphiphilic drug, diclofenac diethylamine (DDA), and adjusted the formulation pH to >7.2. At this pH, the completely ionized drug (pKa 4.87) in w/o system remained mostly entrapped in inner aqueous phase and released very slowly. However, for the same system, o/w emulsions (ie, systems containing higher proportion of aqueous phase) would release the drug at a faster rate due to the drug being in external phase (Figure 1). As most of the drugs are weak acid or base form, formulation pH often governs the release rate by influencing lipo/hydrophilicity of the drug dissolved in microemulsion.
The surfactant layer surrounding the droplets would also have a major impact on the extent of release as the film often plays a crucial role in migration of drug from the inner droplets to aqueous phase. This was demonstrated by modulating release of capric and caprylic acid using different surfactants with different hydrophilicity.21 The nature and volume of internal phase should also be an important consideration. For example, increase in oil phase component of an o/w microemulsion containing doxorubicin resulted in significant decrease in release rate due to formation of more organized, and hence rigid, lamellar structure, which resulted in lower mobility of the drug dissolved in the internal oil phase.22
Selection of drug candidate also plays a very critical role, especially when solubility of the different forms of drug differ significantly. For example, an oil-soluble drug with very high LogP and hence high oil solubility formulated as an o/w microemulsion can be expected to provide a slow and prolonged release of the drug. Salem and Hope demonstrated that using o/w formulations with a morphine base and higher lipophilicity resulted in a more prolonged and consistent exposure compared to morphine hydrochloride salt, which released faster.23
Advances in Development of Prolonged-Release Microemulsions
Controlled-release potential of microemulsions has been explored so far primarily for prolong residence time of drugs administered intravenously as well as to prepare formulations of highly lipophilic drugs, which can be administered as bolus. Such formulations with slow and prolonged exposure also decrease toxicity and improve efficacy. This improvement in PK leading to prolonged exposure has been ascribed to longer circulation time of drug molecules entrapped in oil droplets, which undergo slower uptake and phagocytosis by the reticuloendothelial system (RES).24 These long-circulating oil droplets release the entrapped drug load slowly, resulting in prolonged and higher drug exposure. The consequant improvement in PK and PD can bring significant benefit in cases like postoperative pain in which patients often need to be treated with a PCA morphine pump and are prone to serious adverse events, such as respiratory depression in case of overdosing. Nalbuphine, a morphine-like kappa agonist/partial mu antagonist analgesic, is often used for treating moderate-to-severe pain. It is currently approved as a solution for injection, which can be administered by the IV, IM, or SC route. The short plasma half-life (5 hours) necessitates frequent administration (every 3 to 6 hours). A submicron formulation of nalbuphine and its prodrugs were prepared and subjected to a tail-flick test to evaluate antinociceptive activity after IV administration in tail vein in rats at equimolar dose.24 The formulation doubled the duration of analgesia to 3 hours compared to 1.5 hours from the nalbuphine solution.
OPPORTUNITIES
Recurrent pain in several conditions demands repeated oral or parenteral dosing. However, available therapies do not provide the palliative care in the most acceptable fashion. Oral treatment efficacy demonstrates significant dose-response variability leading to non-response in several cases. For example, proportion of patients who experienced >50% pain relief after 2-week treatment with Ibuprofen and celecoxib were 29% and 30%, respectively, and the number needed to treat were 6.5 and 5.6, respectively.25 While such high variability can be attributed to differences in pain threshold, disease severity, etc, poor and variable oral absorption resulting from difference in drug disposition during gastro-intestinal dissolution, absorption, gut wall metabolism, and hepatic clearance also play a significant role. This is evident from the fact that the lower bioavailability (22% to 40%) and 10-fold inter-subject variability in the exposure of celecoxib, the most widely used Cox-2 selective agent.26,27 Such variability often fails to result in satisfactory pain relief in moderate-to-severe pain incidences, prompting use of injectable formulations which result in fast onset and more predictable treatment outcome. However, injectables have their own set of problems. Frequent injectable treatment inflicts pain, results in frequent clinical visits, or worse, demands in-patient treatment. Injectable formulations that provide prolonged release promise to bring significant benefit in such conditions. However, available technologies do not support development of such formulations due to challenges associated with complex development and manufacturing procedures, limited excipient and process options, as well as high cost of finished product. Microemulsions offer significant opportunities in this area. With several drugs being highly lipophilic, and hence difficult to formulate as injectables, microemulsions can be used to successfully develop injectable formulations by dissolving such drug in the oil phase. While such simple-to-develop formulations can be used similar to fast-acting IV injectable formulations (eg, Microfol) without using toxic co-solvents, by careful designing of formulation as previously described, in vitro and in vivo release of entrapped drug can be modulated to increase duration of analgesia. An SC or IM formulation would further prolong the duration of drug exposure, and can potentially sustain the drug release beyond 24 hours.
CONCLUSION
Improved, prolonged-release formulations for palliative care of patients in in-patient and out-patient care are a widely acknowledged need that has not been met. As per an IASP report, even after major advancements, 50% of patients have severe or intolerable pain after surgery, which increases risk of persistent pain after surgery.28 Currently approved products often lack consistent efficacy and/or result in severe side effects or add a significant dosing burden. Due to these reasons, patients often discontinue the treatment or are undertreated, which results in significant social, clinical, and economic burdens.29,30 While there is general consensus on the need of better drug delivery, available technologies often fail to support the development. Microemulsion technology needs more investigation for developing such formulations. With its various features, it is very likely to facilitate development of prolonged-release injectable analgesic formulations.
To view this issue and all back issues online, please visit www.drug-dev.com.
REFERENCES
1. General pain fact sheet. http://www.inthefaceofpain.com/content/uploads/2011/12/factsheet _Pain.pdf. Accessed Sept. 10, 2013.
2. WHO’s pain ladder for adults. http://www.who.int/cancer/palliative/painladder/en/. Accessed Sept. 6, 2013.
3. Post-operative pain management. In: Guidelines on pain management. Agency for healthcare research and quality, US department of health & human services. http://www.guideline.gov/content.aspx?id=23897#Section420. Accessed Sept. 6, 2013.
4. Karasulu HY. Microemulsions as novel drug carriers: the formation, stability, applications and toxicity. Expert Opin Drug Deliv. 2008;5:119-135.
5. Li G, Fan Y, Li X, et al. In vitro and in vivo evaluation of a simple microemulsion formulation for propofol. Int J Pharm. 2012;425:53-61.
6. Darole PS, Hegde DD, Nair HA. Formulation and evaluation of microemulsion based delivery system for amphotericin B. AAPS PharmSciTech. 2008;9:122-128.
7. Nakhare S, Vyas SP. Preparation and characterization of multiple emulsion based systems for controlled diclofenac sodium release. J Microencapsul 1996;13:281-292.
8. Fosnocht DE, et al. Patient preference for route of pain medication delivery. J Emerg Med. 2004;26:7-11.
9. Beel TL, et al. Patient preferences regarding pain medication in the ED. Am J Emerg Med. 2000;18:376-380.
10. Gan TJ, et al. Patient preferences for acute pain treatment. Br J Anaesth. 2004;92:681-688.
11. Rupp T, Delaney KA. Inadequate analgesia in emergency medicine. Ann Emerg Med. 2004;43:494-503.
12. Garbed RO, et al. Pain after discharge: A pilot study of factors associated with pain management and functional status. J Emerg Nurs. 2006;32:288-293.
13. Prince LM. Emulsion and emulsion technology, Part I. New York: Marcel Dekker Inc. 1974.
14. Ke WT, et al. Physical characterization of microemulsion systems using tocopheryl polyethylene glycol 1000 succinate(TPGS) as a surfactant for the oral delivery of protein drugs. J Control Release. 2005;102:489-507.
15. Ottinger I. Microemulsion preconcentrate comprising a renin inhibitor. WO2005058291 (2005).
16. Bagwe RP, et al. Improved drug delivery using microemulsions: rationale, recent progress, and new horizons. Crit Rev Ther Drug Carrier Syst. 2001;18:77-140.
17. Li G, et al. In vitro and in vivo evaluation of a simple microemulsion formulation for propofol. Int J Pharm. 2012;425:53-61.
18. Schulz MB, Daniels R. Hydroxypropylmethylcellulose (HPMC) as emulsifier for submicron emulsions: influence of molecular weight and substitution type on the droplet size after highpressure homogenization. Eur J Pharm Biopharm. 2000;49:231-236.
19. Darole PS, et al. Formulation and evaluation of microemulsion based delivery system for amphotericin B. AAPS PharmSciTech. 2008;9:122-128.
20. Djordjevic L, et al. In vitro release of diclofenac diethylamine from caprylocaproyl macrogolglycerides based microemulsions. Int J Pharm 2005;296:73-79.
21. Washington C, Evans K. Release rate measurements of model hydrophobic solutes from submicron triglyceride emulsion. J Control Release 1995;33:383-390.
22. Formariz TP, et al. Relationship between structural features and in vitro release of doxorubicin from biocompatible anionic microemulsion. Colloids Surf B Biointerfaces. 2007;60:28-35.
23. Salem A, Hope W. Absorption of morphine from a slow-release emulsion used to induce morphine dependence in rats. J Pharmacol Toxicol Methods. 1998;40:159-164.
24. Wang JJ, et al. Submicron lipid emulsion as a drug delivery system for nalbuphine and its prodrugs. J Control Release. 2006;115:140-149.
25. Moore RA, et al. Responder analysis for pain relief and numbers needed to treat in a meta-analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Ann Rheum Dis. 2010;69:374-379.
26. Paulson SK, et al. Pharmacokinetics of celecoxib after oral administration in dogs and humans: effect of food and site of absorption. J Pharmacol Exp Ther. 2001;297:638-645.
27. Summary of product characteristics: Celebrex, http://www.medicines.org.uk/emc/ medicine/14534. Accessed Sept. 10, 2013.
28. Guideline for the management of pain in adults. The College of Emergency Medicine. London. June 2010. http://www.iasppain. org/AM/Template.cfm?Section=PressRelease&Template=/C M/ContentDisplay.cfm&ContentID=2908. Accessed Sept. 6 2013.
29. Gustavsson A, et al. Socio-economic burden of patients with a diagnosis related to chronic pain-register data of 840,000 Swedish patients. Eur J Pain. 2012;16:289-299.
30. Phillips CJ. Economic burden of chronic pain. Expert Rev Pharmacoecon Outcomes Res. 2006;6:591-601.

Dr. Rajesh Dubey is presently working with Dr. Reddy’s Laboratories Ltd (Associate Director, Business Development), where he is involved in the conceptualization and development of proprietary formulations. He is also a visiting lecturer at King’s College London from where he completed his post-doctoral studies in pharmacy.

Dr. Luigi G. Martini is the CEO of Rainbow Medical Engineering Ltd, a specialist ultrasonic welding and medical device fabrication company and appointed Professor of Pharmaceutical Innovation at King’s College London, where he is the UK’s first and only Industrial Pharmacist teaching practitioner providing an important link between Industry and Academia. His research interests include personalized medicine, drug delivery systems, and medical device engineering. He consults for global companies and regulatory agencies in Europe and the Middle East, recently being invited to participate in European Parliament debates and appointed to the REF2014 sub-panel for Pharmacy, Dentistry, Nursing, and Allied Healthcare professionals in 2011.
Total Page Views: 5977