Issue:November/December 2014
IMMUNOCHEMISTRY ANALYZER MARKET - Mutiplexing Technologies for Infectious Disease, Cancer, Cardiac & Autoimmune Testing Rise Above the Horizon
CURRENT MARKET LANDSCAPE
Immunochemistry analyzers are just now beginning to take the place of clinical chemistry and immunoassay as new or improved assays are being added to the test menus incrementally. According to College of American Pathologists (CAP) product data, an estimated 184,136 immunochemistry analyzers were installed globally in 2013, and recurring consumable sales are a strong factor to segment growth. Only about 17% of these analyzers are installed in the United States, confirming rapid adoption patterns in emerging and rest of the world countries like China, India, and Japan, where there is an untapped opportunity in rural and hospital markets that lack basic diagnostic laboratory infrastructure.
Customer choices exceed combinations of needs in the immunochemistry market, which has more than 100 immunoassay analyzer models. Facing a high degree of competition, manufacturers modulate forecasts by shortening product cycles with new launches or adding value on existing installs.
The US immunochemistry market is also seeing an influx of many other newer companies from Canada, Japan, China, and Europe. The market has undergone a massive change from just a few vendors offering only enzyme-linked immunosorbent assay (ELISA), chemiluminescent immunoassay (CLIA), radioactive immunoassay (RIA), and flourescent immunoassay (FIA) to also multiplex assays, such as microarrays, flow cytometry-based platforms, recombinant immunoassays, and indirect immunofluorescence assays (IFA). Frost & Sullivan end-user analysis also revealed that in the US, some open system platforms are declining in their install base, leading to rapid transition to either FIA or CLIA systems.
MARKET METRICS
Globally, the immunochemistry market represents approximately 40.1% of the global in vitro diagnostics (IVD) market (Figure 1). Immunochemistry is by far the largest segment by volume, mostly due to routine testing. It is primarily driven by growing access to healthcare. Frost & Sullivan estimates that immunochemistry was a $19.9 billion market segment in 2013 and is expected to grow at a compound annual growth rate (CAGR) of 6.8% from 2013 to 2017. The US is a very important and established market for immunochemistry, primarily because it contributes to about $ 5.1 billion of the global immunochemistry market.
Demand for immunochemistry analyzers is slowing in the US and Western and Eastern Europe. Europe’s challenges in this segment include laboratory consolidation in France and late payments from economically troubled Greece, Italy, Spain, and Portugal. China’s growing rural hospital market lacks basic diagnostics laboratory infrastructure and represents an untapped opportunity for affordable immunochemistry analyzers.
INTENSIFYING COMPETITIVE LANDSCAPE
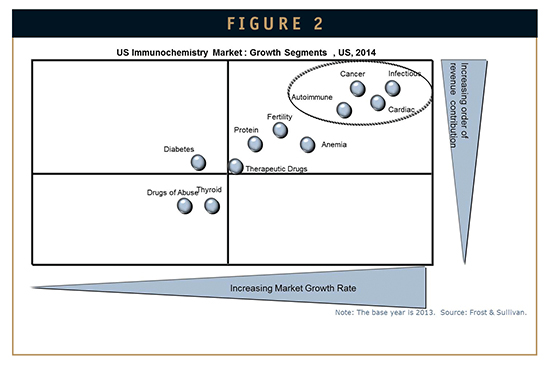
Leading brands, such as Siemens, Abbott Diagnostics, Beckman Coulter, Ortho Clinical Diagnostics, and Roche are focused on high-growth segments, such as infectious disease testing, cancer, cardiac, and autoimmune (Figure 2). Several specialty diagnostic companies are also making their way into the in vitro diagnostics (IVD) market, especially for autoimmune diagnostics. Key companies to pay attention to for innovative diagnostic technologies and products include Inova Diagnostics, SQI Diagnostics, Euroimmune, and Aesku Diagnostics.
WHAT IS DRIVING THE MARKET?
After the severe impact of the Patient Protection and Affordable Care Act (PPACA), there is heavy pressure to reduce healthcare costs. Under such challenging conditions, laboratories continue to look for ways to sustain in such a cost-crunched environment. With cuts in the clinical lab fee schedule (CLFS), cost per test is also falling; hence, it necessitates laboratories to focus more on volume of tests to maintain and sustain the inflow of lab funds. At the same time, there is also heavy pressure for quality, error-free results to ensure patient satisfaction. This forces labs to lean toward high throughput and more automated systems with effective workflow solutions.
Growing volumes due to an increase in the number of insured patients will encourage automation in laboratories. With a deficit in laboratory personnel, managing clinical laboratory flow is becoming difficult. Today’s scenario demands laboratories to seek the help of systems that have a high throughput, owing to growing volumes and also offer remote data acquisition capabilities. Implementing informatics is critical, and the automated analyzers built today offer a full suite for barcode readers, rack detection systems, and sample/plate identification modules to avoid plate or sample switch.
COMPETITIVE FACTORS
Automation and integration, spurred by price and labor pressures inherent in U.S. clinical laboratories, are driving the analyzer market. Offering a broad assay menu, including infectious diseases, is a central competitive factor for increasing installed base of analyzers. Consequently, market participants of this highly competitive market are expected to continue infectious disease immunoassay product development and menu expansion.
Reagent sales typically comprise approximately 85% of fiscal revenues from any single system. Instrument placement is necessary for reagent sales. Therefore, many competitive factors in the immunoassay testing market lie with the instrumentation platform. The installed base of an instrument allows for an increase in test sales. When deciding on a particular testing platform, central laboratories value integrated and automated solutions. The number of tests offered for an instrument promotes the installed base and future reagent sales. A majority of testing platforms are closed systems in which the instrument does not facilitate tests of another provider. The competitive advantage in this market lies with companies that offer a broad testing menu on an integrated and automated system. Servicing the instruments installed in the laboratory is another significant part of the value proposition market participants should offer. Test performance is yet another competitive factor. However, it is becoming difficult for market participants to stand out on the basis of higher sensitivity or other testing characteristics.
COMPETITOR STRATEGIES
As the market becomes highly competitive, it is essential for manufacturers to retain their install base. This provides recurring reagent business, and hence, several market participants adapt to various strategies with their customers, such as:
Offering Large Discounts on the List price of the Analyzer – As the market for immunoassay and clinical assay systems is enormously shrinking and eroding, companies such as Roche are now adding immunoassay components or clinical chemistry systems to existing analyzers in order to make it an integrated analyzer. This allows the customer groups. As a result, customers receive large discounts. This works in in their favor when laboratories have stringent budgets.
Bundle Reagent Rental Contracts – The tendency toward opting for reagent rentals varies from region to region. For example, laboratories in emerging markets will opt for multiple reagent vendors, while established markets, such as laboratories in the US and Europe, prefer to select the instrument and the reagent from the same vendor, ensuring compliance and accreditation standards.
Offering Upgrades & Add-On Analyzers & Components – In order to retain their install base, many vendors offer upgrades to existing analyzers at a very low cost to increase capabilities of existing outdated analyzers.
SUMMARY
The key challenge when building on install base is the high cost associated with transition. Even when dissatisfied, most lab managers endure outdated instruments to avoid resource-intensive issues, such as the purchase process, staff retraining, protocol standardization, and data management system reevaluation. As labs consolidate, the immunochemistry analyzer market relies on instrument replacements to generate the needed revenue. Nearly all manufacturers rely on key strategies, such as offering replacements for old clinical chemistry and immunochemistry systems or acquiring clients from other vendors. Competition is stiffening as most vendors face difficulties increasing their customer base. The future will see CLIA as a growing analyzer segment with tremendous focus on areas, such as infectious disease testing, and autoimmune and oncology segments. The market is moving toward multiparametric assays, and many companies are exploring the use of multiplex technologies using protein and peptide arrays for autoimmune diagnostics.
To view this issue and all back issues online, please visit www.drug-dev.com.

Divyaa Ravishankar is a Senior Industry Analyst for Frost & Sullivan’s Life Sciences practice. She has diverse expertise within healthcare IT and life sciences with a focus on in vitro diagnostics. Her expertise constitutes of laboratory research and management consulting. To supplement her expertise, she also has broad-ranging industry experience in varying sectors in which she has established long-standing working relationships with leading industry participants in areas like clinical diagnostics, pharmaceuticals, and biotechnology. Ms. Ravishankar earned her Master of Science (Hons.) in Biological Sciences from Birla Institute of Technology and Science, Pilani, India. For more information on Frost & Sullivan’s global Life Sciences practice and offerings, please email jennifer.carson@frost.com or visit www.frost.com.
Total Page Views: 5304