Issue:January/February 2016
IMMUNOASSAY PLATFORM - Accelerating Drug Development & Clinical Validation With Single Molecule Counting
INTRODUCTION
Ultrasensitive Single Molecule Counting (SMCTM) technology provides an indispensable tool in the researcher’s arsenal to help move novel biology forward, fueling the discovery and development of new therapeutics. This technology is now available to leading pharmaceutical R&D laboratories, clinical research organizations (CROs), and academic institutions around the world through the Research-Use-Only Erenna® Immunoassay System, immunoassay kits, reagents, and custom services.
Enzyme Linked Immunosorbent Assays (ELISAs) are the traditional approach to protein biomarker quantification due to target specificity and ease of operation. However, ELISA methods often fail to quantify the target of interest if present in low abundance, which leads researchers to create more complex studies to produce the data they need, change their sample matrix, or stop investigating a putative marker entirely. Addition of patented digital SMC technology to the traditional immunoassay workflow enables detection of low-abundance, previously undetectable biomarkers, such as proteins and nucleic acids, with unparalleled sensitivity and accuracy, capturing concentrations down to the femtogram/mL level. This combination of digital counting and standard immunoassay processing allows quantification of biomarkers at sensitivities of 10- to 1,000-fold over other commercially available technologies.
With the addition of SMC technology to plate- and bead-based immunoassay formats, researchers and clinicians can now detect and monitor changes of established disease biomarkers that are present at extremely low levels, such as cardiac troponin I and cytokines. New molecular insights can be gained and greater utility can be achieved with disease biomarkers, such as those for cardiovascular, Alzheimer’s, Parkinson’s, rheumatoid arthritis, Crohn’s disease, certain cancers, and inflammatory- and autoimmune-based diseases. Even the smallest changes in biomarker levels can be measured, allowing researchers to gain unprecedented insights into complex disease biology, drug efficacy, and drug safety, and providing clinicians with a broader assessment of patient risk to enable proactive health management.
HOW IT WORKS
The Singulex® platform couples SMC technology with robust microparticle-based immunoassays to provide higher sensitivity and broader dynamic range over traditional immunoassay and ELISA platforms (Figure 1). The steps unique to SMC technology include concentrating the detection area (improves signal) and removing the detector from the assay plate (reduces background). This results in reproducible signal at low analyte concentrations, and better quantification of proteins, including those at very low abundance.
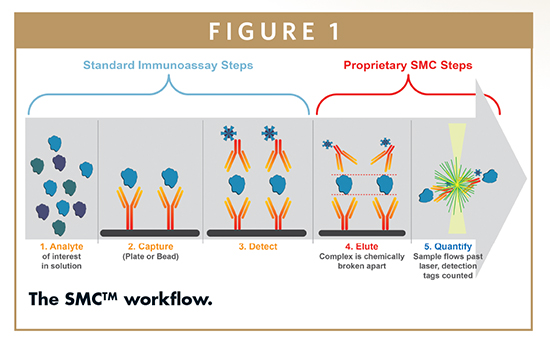
During the capture and detection steps, specific antibodies translate each biomarker into a signal. During final elution, fluorescent dye-labeled detection antibodies are released from the immune complexes and separated magnetically from the paramagnetic microparticles. These detection antibodies are the source of signal. The Erenna® Immunoassay System uses a robust digital SMC module to count photons, allowing precise measurement of low-abundance biomarkers with high statistical confidence. The instrument’s capillary tube contains a very small interrogation space that is illuminated by a laser. Single fluorescently labeled molecules are detected as they generate intense flashes of light when passing through the interrogation space. Detected signals with peak intensity above the threshold of background fluorescence are counted as digital events. The instrument also records the sum of all digital events counted. At high concentrations, a proprietary algorithm computes the total sum of all photons recorded. Thus, the SMC technology improves assay sensitivity and extends dynamic measuring range one to three orders of magnitude greater than possible with traditional immunoassay technologies (Figure 2).
The SMC process enables digital detection at the lower end of the dynamic range. The signal is specific to individual antibodies passing through the confocal laser point. This decreases the background and reduces the lower limit of quantification. At the upper end of the range, the signal is more analog, and the instrument uses a proprietary algorithm and total photons to measure against a standard curve.
IDENTIFYING CLINICALLY RELEVANT BIOMARKERS
Several considerations must be taken into account when establishing a biomarker for translational research, including the demonstration of disease specificity, the ability to measure the biomarker in “normal” healthy states, demonstration of low biological variability, as well as the ability to predict future disease and/or clinical outcomes. Traditional technologies often lack the requisite specificity and sensitivity to address these critical considerations, presenting challenges to establishing the clinical utility of a potential biomarker.
SMC technology can help to overcome the many challenges associated with the biological qualification of protein biomarkers for clinical and translational research. With improved assay precision and sensitivity, SMC technology has enabled the measurement of endogenous biomarker concentrations at femtogram/mL levels and precise monitoring over time. For example, this technology has established high definition cardiac troponin I (cTnI) as a physiologically relevant biomarker for cardiovascular disease (CVD) risk characterization and chronic disease management.
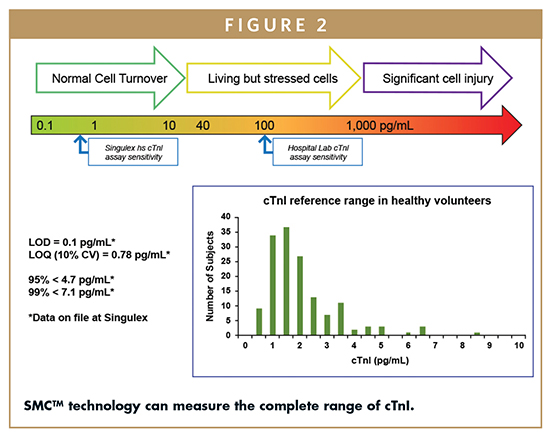
cTnI is a contractile protein that is specific to cardiac monocytes and is released at very low levels through normal cell turnover (0.1 to 10 pg/mL). However, damage to the cardiac monocytes during acute myocardial infarction (AMI) releases cTnI into the circulation at significantly higher levels (1,000 pg/mL +). The early identification of AMI is vital for limiting myocardial damage and preserving cardiac function. According to the Minnesota Heart Survey and Framingham Heart Study, cTnI predicts cardiovascular (CV) death and the gradient of risk for heart failure (HF) in primary prevention, respectively. This biomarker also predicts CV death and HF in secondary prevention. Therefore, the ability to measure the full range of cTnI and monitor changes over time enables the use of this biomarker in clinical research studies of chronic disease management.
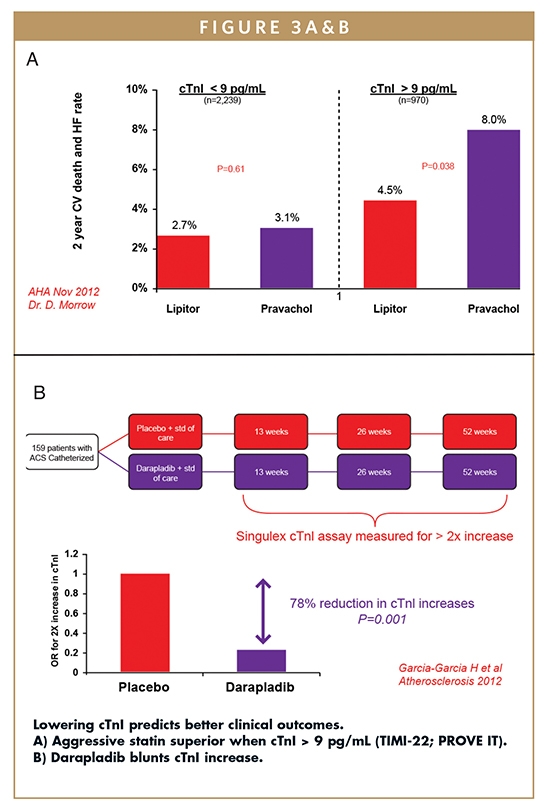
SMC technology can measure the complete range of cTnI concentration in circulation with a sensitivity of 0.1 pg/mL, allowing this biomarker to stratify clinical research subjects for immediate vs. longer term risk of cardiovascular disease (Figure 2). In addition, low biological variability of this biomarker combined with high precision of the assay allows for monitoring of disease progression as well as response to therapy (Figure 3). cTnI is a relevant cardiac biomarker that can be used to manage and monitor subjects of clinical research through the use of SMC technology.
COMPLETE PK/PD PROFILING
The use of SMC technology also offers new perspectives for low therapeutic index drug development. Traditional methodologies offer limited capacity for pharmacokinetic/pharmacodynamic (PK/PD) profiling and are often unable to show the full clearance profile of some drugs. In addition, they often have limited ability to measure the low concentrations of a given drug, such as used in micro-dosing studies. New digital high-definition immunoassays allow for the detection of lower levels of a drug to provide a more complete PK/PD profile, as well as allow for the identification of unpredictable clearance patterns undetectable by traditional immunoassay methodologies. These data can inform Go/No-Go decisions in Phase I/II of drug development and thereby reduce attrition and development costs.
Interleukin-13
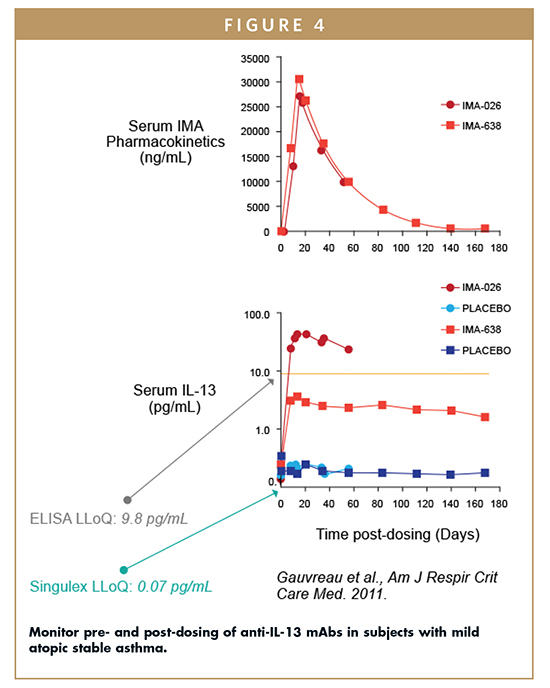
IL-13 is a Th2 cytokine implicated in asthmatic inflammation, and anti-IL-13 therapeutics are in development to counter autoimmune diseases. Accurate measurement of circulating IL-13 levels is therefore needed for PK/PD and mode of action (MOA) studies to support these drug development efforts. However, IL-13 quantitation requires greatly improved sensitivity over traditional immunoassay or ELISA techniques, as circulating levels of this molecule are ≤ 1 pg/mL.
Improved assay sensitivity has been achieved using SMC technology, which offers up to a 140-fold improvement in the lower limit of quantification (LLoQ; pg/mL) over traditional ELISA methods. Anti-IL-13 monoclonal antibodies, IMA-026 and IMA-638, each of which competes with different receptors for IL-13 binding, were evaluated for anti-IL-13 treatment in patients with mild atopic stable asthma. The Singulex proprietary IL-13 assay (LLoQ = 0.07 pg/mL), but not traditional ELISA (LLoQ = 9.8 pg/mL), enabled quantification of baseline measurements of the clinical study population (n=182). All 99th% cut-off values for healthy and asthmatic subjects at baseline were less than 1 pg/mL. The assay also enabled longitudinal measurement of serum IL-13 levels from antibody- and placebo-treated subjects (Figure 4). An ELISA assay would be unable to measure the full profile for antibody- or placebo-treated subjects.
Serum cTnI
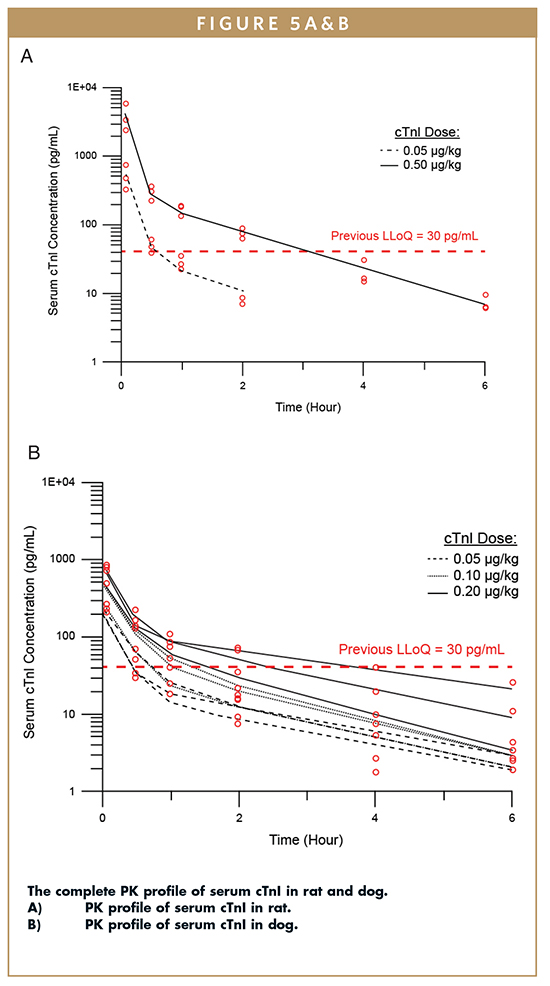
The effect of minor serum cTnI elevations, independent of extensive cardiomyocyte damage, has not been evaluated thoroughly because traditional assays (LLoQ ~30 pg/mL) have not been sensitive enough for this purpose. Transient or slight cardiomyocyte damage may not generate a large and persistent release of cTnI, making it difficult to identify a treatment-related transient increase using limited sampling times. However, recent evidence suggests that even small elevations of serum cTnI above baseline are correlated with an increased risk of myocardial-related mortality in humans. Therefore, the ability to establish and monitor baseline concentrations of serum cTnI in the rat or dog is essential to assessing the cardiovascular safety of therapeutic compounds in development.
The pharmacokinetics of cTnI in the Wistar Han rat and beagle dog was analyzed with a two-compartment model; blood samples were collected at multiple time points, and cTnI concentration was measured (Figure 5). For each animal model, single-digit pg/mL baseline concentrations of cTnI were detected, which were previously undetectable by other cTnI assays. Biphasic disposition of serum cTnI was observed after intravenous (IV) injection. The complete cTnI profile was exposed using select low dose cTnI concentrations estimated to be equivalent to cTnI levels expected after slight cardiomyocyte damage. The cTnI levels observed fall below the LLoQ for previous assays within 1 hour of dosing.
SUMMARY
Traditional ELISA methodologies have limitations in sensitivity and quantification, sample volume requirements, dynamic range, and matrix effects. These factors reduce the utility of the traditional microplate-based immunoassay for sample stratification, endogenous level quantification, and determination of appropriate dilutions for sample measurements. Minimal changes made to a traditional ELISA assay that incorporates SMC technology can make the difference between a particular biomarker being undetectable and being a viable research target. In addition, SMC technology accelerates biomarker research by providing femtogram/mL biomarker detection across multiple disease areas and drug states.
The use of SMC technology enables researchers to attain greater sensitivity, use less sample, and, in some cases, reduce costs, while still retaining the ease of use and familiarity of the traditional ELISA method. The performance improvement can allow, for example, the tracking of small changes in previously undetectable analytes to better understand disease progression and stratify patient populations, to gain insights into novel biological mechanisms, and/or to employ better biotherapeutic monitoring strategies, such as microdosing for assessing safety and efficacy.

Dr. Steven Suchyta is a Senior Global Product Manager at MilliporeSigma, the life science business of Merck KGaA, Darmstadt, Germany. He is responsible for all of the company’s single protein detection immunoassay technologies, including Singulex SMCTM technologies, Gyromark HTTM assay, and ELISA kits. Dr. Suchyta earned his BS and MS from The University of Georgia and his PhD in Genetics from Michigan State University.
Total Page Views: 6651









