Issue:April 2015
GASTRORETENTIVE DELIVERY - Box-Behnken-Designed Gastroretentive Floating Tablets of Famotidine
ABSTRACT
The aim of this study was to develop and characterize a single-unit, floating controlled drug delivery system of famotidine hydrochloride using a blend of natural polymer (xanthan gum) and synthetic polymer (hydroxypropyl methylcellulose [HPMC]) along with a gas-generating agent (sodium bicarbonate) by applying Box-Behnken design. Famotidine hydrochloride floating tablets were prepared by direct compression technique. Tablets were evaluated for physical characteristics viz. hardness, swelling index, floating capacity, weight variation, and in vitro drug release. All tablets floated for more than 12 hrs in 0.1 N HCl at 37°C ± 0.5°C, and the in vitro drug release was found to be vary from 71% to 84%. The percent release was maximum at low value of HPMC, medium value of xantham gum, and high value of sodium bicarbonate. A mathematical model was developed to formulate floating tablets of famotidine. The data fitting to Koremeyer-Peppas equation revealed that the release mechanism from the dosage form followed the non-fickian transport. These tablets will enhance patient compliance and improve patient’s quality of life.
INTRODUCTION
Residence time of an administrated dosage form in the stomach is generally short due to rapid gastric emptying, and thereby frequency of administration has to be increased for the desired pharmacological action.1 Fluids taken at body temperature leave the stomach faster than colder or warmer fluids. Fast gastrointestinal transit could result in incomplete drug release from the device above the absorption zone leading to diminished efficacy of the administered dose. Therapeutic efficiency of drugs that are well absorbed in the stomach, such as amoxicillin, may be increased with a longer residence time. In addition, the bioavailability of drugs that are unstable in the colon, such as ranitidine, as well as drugs that exhibit poor solubility in the intestinal tract, such as diazepam, could be increased by extending the drug residence time in the stomach.2
The classification of different modes of gastric retention has been listed by Hwang et al and Bardonnet et al.3,4 An interesting approach to provide floating drug delivery systems is based on the formation of carbon dioxide within the device upon contact with body fluids. Upon contact with acidic aqueous media, carbon dioxide is generated and entrapped within the gelling hydrocolloid, causing the dosage form to swell and hence making the system buoyant.5
The blends of two different kinds of polymers, ie, natural and synthetic, are being used for floating drug delivery. HPMC is a stable material, although it is hygroscopic after drying. Xanthan gum, on the other hand, forms a highly viscous solution in warm water, but alone, it is unable to make tablets that are buoyant enough to make tablets float. However, the combination of these two polymers (along with sodium bicarbonate) provides optimum viscosity and porosity to make the tablets float.
Blends of polysaccharides and cellulose ethers can be used to modulate drug-release profiles not achievable by the use of either type of polymer.6 HPMC K4M powder is a stable material, although it is hygroscopic after drying. Aqueous solutions are comparatively enzyme-resistant, providing good viscosity stability during long-term storage. Chemically, it is cellulose hydroxypropyl methyl ether. Functionally, it is used as coating agent, film-former, rate-controlling polymer for sustained release, stabilizing agent, suspending agent, tablet binder, and viscosity-increasing agent. Xanthan gum is widely used as gelling agent, suspending agent, sustained-release agent, and viscosity-increasing agent.7 The aim of the work is to develop gastroretentive floating tablets of famotidine hydrochloride applying Box-Behnken design using a blend of natural and synthetic polymers via an effervescence technique. The objective behind the study was to analyse the effect(s) of Conc. of HPMC K4M (X1), Conc. of xanthan gum (X2), and Conc. Of Sodium bicarbonate (X3) on the release of famotidine hydrochloride.
MATERIALS
Famotidine hydrochloride was obtained as a gift sample from Synmedic Laboratories, India. HPMC K4M was supplied by Colorcon Asia Pvt. Ltd., India, as a gift sample. Xanthan gum and sodium bicarbonate were purchased from Loba Chemie, Mumbai, India. All other ingredients were of analytical grade and were used as received.
METHODS
Pre-Compression Studies
Angle of Repose – The weighed powder blend was transferred in the funnel, and the height of the funnel was adjusted in such a way that the tip of the funnel just touched the apex of the powder blend. The powder blends flow through the funnel freely on to the surface. The diameter of the powder cone was measured, and the angle of repose was calculated using the following equation: tan θ = h/r. Where h and r are the height and radius of the powder cone.
Bulk Density (BD) & Tapped Density (TD) – The 2 g of powder blend, previously shaken to break any agglomerates formed, was transferred to bulk density apparatus. The initial volume was noted, and the cylinder was tapped until no further change in volume was noted. BD and TD are calculated using the following equations: BD=Weight of the Powder Blend/Untapped Volume of the Packing; TD=Weight of the Powder Blend/Tapped Volume of the Packing.
Compressibility Index – This was determined by Carr’s compressibility index. It is a simple test to evaluate the BD and TD of a powder and the rate at which it packed down. The formula for Carr’s Index is: Carr’s Index (%) = [(TDBD) x100]/TD.
Box-Behnken Design
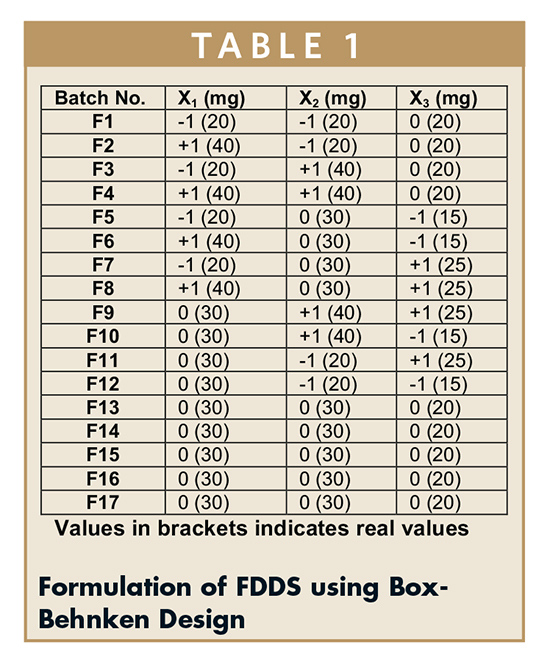
Floating tablets of famotidine hydrochloride were formulated according to the Box-Benkhen Design. To evaluate three factors (n) at three levels (k), the Box-Behnken design consisted of seventeen batches (F1 to F17). Factors with levels and formulations are shown in Table 1.
Preparation of Directly Compressed Tablets
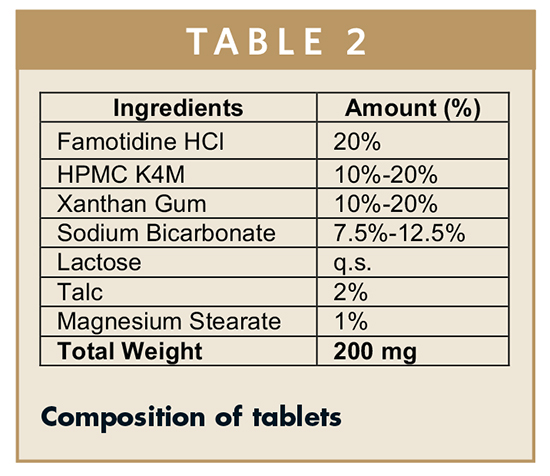
Tablets were prepared using the direct compression technique. Famotidine hydrochloride, HPMC K4M, xanthan gum, sodium bicarbonate, and lactose were weighed (Table 2) and sifted through a No. 40 mesh sieve and mixed well to form a uniform mass. Talc and magnesium stearate were sifted through a No. 60 mesh and added to the blend and mixed well. Final blend was then compressed into tablets using 8 mm round-flat punches. The average total weight of tablet was 200 mg.
Evaluation of Floating Tablets
Fourier Transform Infra-Red (FTIR) Spectroscopy – It was used to predict any incompatibility or any interaction between the different ingredients in a formulation. FTIR spectra were recorded using Alpha-Brucker, FTIR Spectrophotometer, Brucker, Germany. The spectra were recorded over a range of 400 to 4000 cm-1.
Buoyancy: Floating Lag Time & Floating Time – Floating properties were examined following the procedure reported by Baumgartner et al.8 Briefly, the tablets took to come to the water surface (floating lag time) and the time the tablets constantly float on the water surface (duration of floating) were evaluated in a dissolution vessel filled with 900 ml of either de-ionized water or 0.1 N HCl (pH 1.2) at a temperature of 37°C ± 0.5°C at a rotation speed of 50 rpm. The measurements were carried out for each series of tablets (n=3).
Determination of Swelling Index – The swelling index (SI) of tablets was determined in 0.1N HCl at room temperature. The swollen weight of the tablet was determined at predefined time intervals. The swelling index was calculated by the following equation 2: Swelling Index = (W2-W1)*100/W1. Where W1 is the initial weight of tablet, and W2 is the weight of tablet at time t.9
In Vitro Drug Release – In vitro drug release testing from tablets was conducted using a USP Type-II Dissolution Apparatus (Paddle type) (Labindia, India). The dissolution media used for release testing of tablets was 900 ml of 0.1N HCl maintained at 37°C ± 0.5°C and agitated at 50 rpm. Samples of 10 ml were withdrawn at predetermined intervals, were replenished by fresh medium, and analysed using a UV spectrophotometer at 265 nm. All measurements were performed in triplicate. For the release data analysis, cumulative percent drug release versus time (zero-order kinetics), the log cumulative percent drug remaining versus time (first order kinetics), cumulative percent drug release versus the square root of time (Higuchi kinetics), and log cumulative percent drug release versus log time (Korsmeyer-Peppas kinetics) was plotted.
Statistical Analysis of the Data & Validation of the Model – Response surface modelling and evaluation of the quality of fit of the model for the current study were performed employing Design Expert® software (Version 8.0.7.1, Stat-Ease Inc., Minneapolis, MN). The models were generated for all the response variables using multiple linear regression analysis. 3D response plots were constructed using Design-Expert software.10
RESULTS & DISCUSSION
Physical Characterization of Powder Blend
The powder blend was evaluated for flow properties. Bulk density of powder blend was found between 0.365 to 0.412 g/cm3, and Tapped density ranged between 0.419 to 0.486 g/cm3. Carr’s index was found to be in the range of 12.59 to 16.52, indicating good flow. Hausner’s ratio values for all the formulations were found to be about 1.2, indicating low interparticle friction. Angle of repose was found to be in the range of 25.40 to 29.40. The values of angle of repose were less than 30, indicating good flowability. These values indicate the prepared blend exhibited good flow properties.
Fourier Transform Infra-Red (FTIR) Spectroscopy
To study the incompatibility between drug and polymer, FTIR spectra of all ingredients were recorded over a range of 500 to 3500 cm-1. The FTIR spectrum of famotidine shows characteristic absorption bands at 3450, 2900, 1645, 1605, 1547, 1293, and 1136 cm-1. FTIR of mixture showed the presence of polymers and drug with no shifting in the peaks, indicating there was no significant interaction between drug and polymers (Figure 1).
Evaluation of Floating Tablets
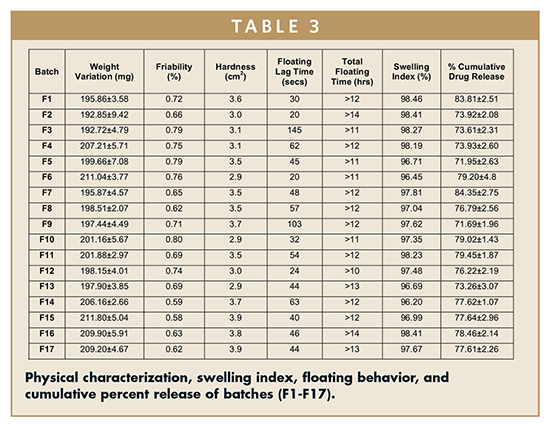
Weight Variation, Friability & Hardness – Weight variation was within limits as prescribed in USP (7.5%). Hardness of the prepared tablets ranged between 2.9 to 3.9 kg/cm2. Friability was found to be less than 1%. Results are shown in Table 3.
Floating Behavior – All the formulations consistently floated for more than 12 hrs, while the floating lag time varied from 20 to 103 seconds. Tablet batches with high polymer content have large lag time (> 100 secs) compared with the batches having medium polymer content (30 to 50 secs).
Swelling Index – Tablets composed of polymeric matrices build a gel layer around the tablet core upon coming in contact with water. This gel layer governs and affects the drug release. To obtain floating, the balance between swelling and water acceptance must be restored.11 Swelling index values start decreasing when polymer erosion starts in the medium. Combination of HPMC K4M and xanthan gum resulted in a higher swelling index varying from 96% to 99% (Table 3).
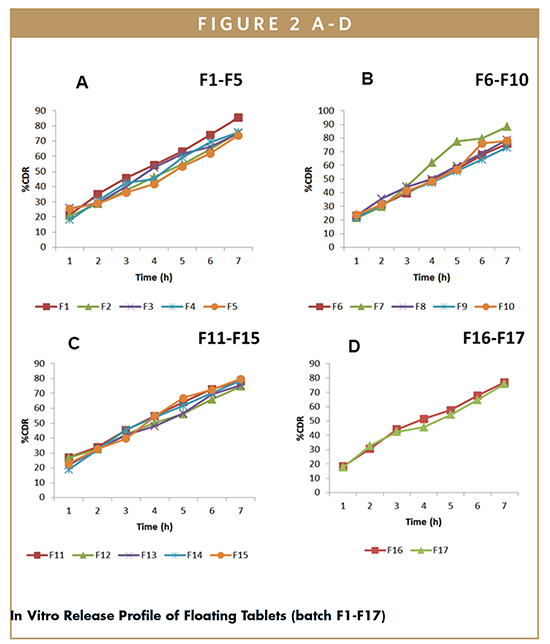
In Vitro Drug Release – The percentage cumulative drug release of all batches of floating tablets (F1- F17) was determined for 7 hrs (using Dissolution Data Solver software), and were found to vary from 71.69% ± 1.96% to 84.35% ± 2.75% (Table 3). Batch F9 shows lowest %CDR (71.69%), whereas batch F7 shows highest %CDR (84.35%). The in vitro release profiles of all the batches (F1-F17) are shown in Figure 2.
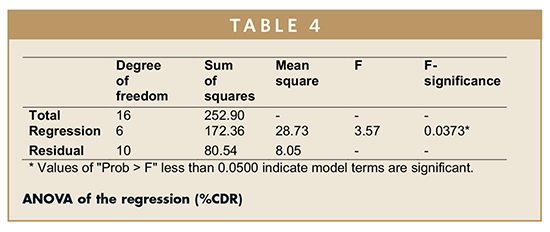
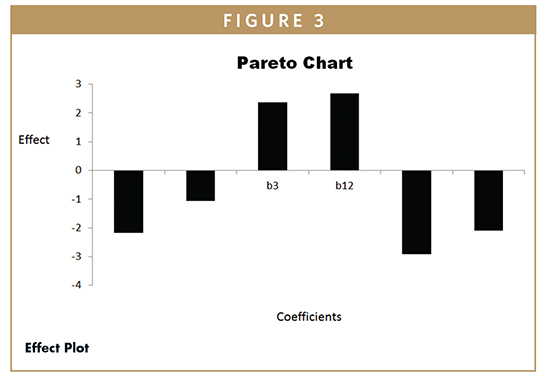
ANOVA on Percentage Cumulative Drug Release From Various Formulations – Batch F9 shows lowest %CDR (71.69%), whereas batch F7 shows highest %CDR (84.35%). The percent release was maximum at a low value of HPMC, mid level of xanthan gum, and high levels of sodium bicarbonate. The %CDR increases with increase in amount of sodium bicarbonate to ensure complete effervescence of the tablets. However, low amounts of both polymers are required for increase in %CDR. The effect of various coefficients on %CDR is shown in Figure 3. On the basis of %CDR Floating lag time and swelling index, batch F1 was selected as the optimized batch due to floating lag time of 30 secs and 83.81% CDR. ANOVA was applied on %CDR to study the fitting and significance of model (Table 4). The model developed from multiple linear regression to estimate effect (Y) can be presented mathematically as:
Y = 77.581 – 2.171 X1 – 1.062X2 + 2.373 X3 + 2.680 X1 X2 – 2.923 X1 X3 – 2.100 X2X3. Where Y = Cumulative percent drug release; X1 = amount of HPMC K4M; X2 = amount of xanthan gum and X3 = amount of sodium bicarbonate.
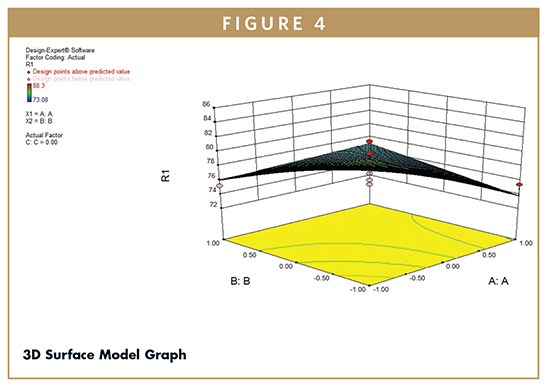
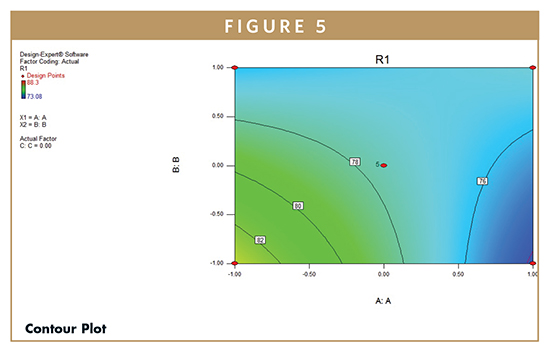
F-test was carried out to compare the regression mean square with the residual mean square. The ratio F = 3.57 shows regression to be significant. The estimated model, therefore, may be used as response surface for the %CDR as shown by 3D Surface (Figure 4) and Contour plots (Figure 5) employing Design Expert software (Version 8.0.7.1, Stat-Ease Inc., Minneapolis, MN). The developed model can further be utilized to determine the desired %CDR. Figures 4 and 5 display the 3D surface and contour plot of cumulative percent of drug release as a function of formulation variables. The results show that the in vitro drug release was found to be varying from 71% to 84%. The percent release was maximum at low value of HPMC, medium value of xantham gum, and high value of sodium bicarbonate.
Kinetics of Drug Release – The dissolution data of batches F1 to F17 was fitted to zero-order, first-order, Higuchi, Hixson-Crowell, and Korsmeyer-Peppas models using Dissolution Data Solver software. The values of correlation coefficient (R2) were used to select the most appropriate model. The release profile of the best batch, F1, fitted best to the Korsmeyer-Peppas model (R2 = 0.9984). Thus, it may be concluded that drug release from gastroretentive famotidine hydrochloride tablets is best explained by the Korsmeyer-Peppas model. Analysis of release data as per Peppas equation showed value of release exponent 0.718, ie, it falls in the range of 0.5 to 0.89, indicating non-fickian (anomalous) diffusion as the drug-release mechanism. Anomalous transport includes both polymer erosion and polymer swelling (relaxation).
CONCLUSION
In the present study, a gastroretentive floating drug delivery system of famotidine hydrochloride was formulated to improve its gastric residence time. The Box-Behnken design was applied to study the effect of formulation variables (amount of HPMC K4M, xanthan gum, and sodium bicarbonate) on drug release, floating, and swelling properties of the tablets of famotidine hydrochloride. All the formulations were characterized for pre-compression parameters, such as angle of repose, bulk and tapped density, and compressibility index. These batches were further characterized for swelling index, buoyancy, hardness, friability, and in vitro dissolution. The data from the release profile were fitted to various mathematical models. The data fitting to the Korsmeyer-Peppas equation revealed that the release mechanism from the dosage form followed the non-fickian transport. Batch F1 was found to be the optimum batch based on the results of buoyancy and %CDR. It can be concluded that the combination of natural and synthetic polymers can be used to formulate the floating tablets. The mathematical model developed in the present study can be used to formulate floating tablets of famotidine with desired buoyancy and percentage release. Retrospectively, this model can be used to design floating tablets of different APIs utilizing the combination of natural and synthetic polymers. A gastroretentive drug delivery system with these floating tablet formulations can reduce dosing frequency, decrease side effects, and improve patient compliance.
To view this issue and all back issues online, please visit www.drug-dev.com.
REFERENCES
1. Arora S, Ali J, Ahuja A, Khar RK, Baboota S. Floating drug delivery systems: A Review, AAPS Pharm SciTech. 2005;6:372-390.
2. Prinderre P. An innovative floating gastroretentive dosage system: formulation and in vitro evaluation. Int J Pharmaceut. 2009;378:23-29.
3. Hwang SJ, Park H, Park K. Gastricretentive drug delivery systems. Crit Rev Therapeut Drug Carrier Sys. 1998;15:234-284.
4. Bardonnet PL, Faivre V, Pugh WJ, Piffaretti JC, Falson F. Gastroretentive dosage forms: overview and special case of Helicobacter pylori. J Controlled Rel. 2006;111:1-18.
5. Yang L, Eshraghi J, Fassihi R. A new intragastric delivery system for the treatment of Helicobacter pylori-associated gastric ulcer: in vitro evaluation. J Controlled Rel. 1999;57:215–222.
6. Elmowafy EM, Awad GAS, Mansour S, El-Shamy AEA. Release mechanisms behind polysaccharides-based famotidine controlled release matrix tablets. AAPS Pharm SciTech. 2008;9:1230-1239.
7. Kim BH, Singh BN. Encyclopedia of Pharmaceutical Technology, IIIrd ed. Marcel Dekker Inc., USA. 2007;1242.
8. Baumgartner S, Kristle J, Vrecer F, Vodopivec P, Zorko B. Optimization of floating matrix tablets and evaluation of their gastric residence time. Int J Pharmaceut. 2000;195:125-135.
9. Srivastava AK, Wadhwa S, Ridhurkar D, Mishra B. Oral sustained delivery of Atenolol from floating matrix tablets – formulation and in vitro evaluation. Drug Dev Indust Pharm. 2005;31:367-374.
10. Shahiwala A. Statistical optimization of Ranitidine HCl floating pulsatile delivery system for chromotherapy of nocturnal acid breakthrough. Eur J Pharmaceut Sci. 2009;37:363-369.
11. Timmermans J, Moes AJ. How well do floating dosage forms float? Int J Pharmaceut. 1990;62:207-211.

Dr. Harish Dureja earned his Master’s from Punajbi University, Patiala, and PhD (Gold Medal) from MD University, Rohtak. He is presently working as Associate Professor in the Department of Pharmaceutical Sciences, Maharshi Dayanand University, Rohtak (India). He has 15 years of experience in teaching and research. He has written 7 invited book chapters/monographs in various publications by international publishers. He has authored more than 90 publications in various national and international journals of repute. Dr. Dureja has been awarded a project for research on anticancer drug delivery, and his current research interests include in silico ADME modelling, nanoparticulate drug delivery, and pharmaceutical regulatory affairs.

Parijat Pandey is working as a research scholar in Industrial Pharmacy in the Department of Pharmaceutical Sciences, MD University, Rohtak. He is working on a funded project entitled Spray Dried NiMS for Orally Disintegrating Tablets.

Mohit Mittal is an alumnus of the Department of Pharmaceutical Sciences, MD University, Rohtak.
Total Page Views: 5173