Issue:March 2018
GAS-POWERED DELIVERY - Innovating Injectable Devices to Deliver Today’s Pharmaceuticals
THE RISE OF BIOLOGICS
The way medications are reaching patients is changing. The advent of biotherapeutics has been driven by their ability to address targets intractable to small molecules, inherently higher target specificity, reduced risk for off-target toxicity, and safety issues. In fact, biologics can be applicable in multiple therapeutic areas and address a variety of targets. With improvements in biomanufacturing processes making biologics more available as well as more cost effective and an increase in approvals, the industry is seeing a corresponding increase in their use for chronic conditions.
To reduce the burden on health services and patients, many medications for long-term illnesses are now administered by the patients themselves at home. The new generation of drug delivery devices designed for biologics therefore have to be easy to use, intuitive, and tailored to the needs of different patient groups.
Self-injection and autoinjection devices, including wearable systems, are an increasingly common way to deliver biologics, and this trend is expected to continue. Valued at $1.7 billion in 2016, the global market for autoinjectors is anticipated to grow at an average rate of 15.1% throughout the next decade, projected to be nearly $7 billion by the end of 2026.1 The increase in chronic diseases and the use of biologics has also been cited as a factor in the increasing value of the global injectable drug delivery market as a whole, which is projected to reach $624.50 billion by 2021 from $362.38 billion in 2016.2
Manufacturers are now making it a priority to fully understand the real-world challenges of patients living with these complex chronic health conditions. In the past decade, autoinjectors have been of increasing interest to biopharma companies developing biologics. Initially used as a device for patients with severe allergies to self-administer epinephrine in emergencies and for military applications, the use of autoinjectors has now broadened into indications such as arthritis and asthma. As new therapeutic areas emerge that lend themselves to self-administration, there are an increasing number of new patient populations in need of autoinjectors.
To address this, pharmaceutical companies and device companies need to work together to develop devices that are adaptable to different patient groups. For example, some patients may not use a device every week, which may lead to challenges around the patient not remembering how to use the device. Autoinjector devices must therefore be intuitive to use and designed with the patient in mind.
WHY IS DELIVERY A CHALLENGE?
Although favored for their efficacy and selectivity, biologic formulations can be challenging to deliver.3 Inhalation, intranasal, and transdermal routes have seen little success, and when taken orally, are inefficient at passing the GI tract.4 Injection is therefore predominantly used as a delivery route; however, the properties of a biologic can cause difficulties for a standard delivery device.
 Patient compliance for injectables is often poor, and this is exacerbated by high regularity of dosing. In an effort to improve compliance, the industry is attempting toreduce the frequency of administrations needed. These efforts include the production of more concentrated formulations, and the identification of more potent APIs, both of which enable more drug to be delivered in the same volume.3With increased concentration comes an increase in viscosity, which creates a number of challenges in delivery via an autoinjector.
Patient compliance for injectables is often poor, and this is exacerbated by high regularity of dosing. In an effort to improve compliance, the industry is attempting toreduce the frequency of administrations needed. These efforts include the production of more concentrated formulations, and the identification of more potent APIs, both of which enable more drug to be delivered in the same volume.3With increased concentration comes an increase in viscosity, which creates a number of challenges in delivery via an autoinjector.
The stability of biotherapeutics often means that a comprehensive supply cold chain is required to ensure that the dose maintains its efficacy. With self-administration, the maintenance of the cold-chain can be variable and potentially lead to reduced efficacy. Therefore, there is a desire to improve the stability of the active ingredient through the use of novel formulation techniques. This can encompass partial structural modification of the biotherapeutic or the use of a more complex formulation. Such attempts to enhance stability can lead to further increases in the viscosity of the resulting formulation. In fact, it is difficult to evaluate true viscosity as temperature is such a key factor.
Traditional spring–based technologies for autoinjectors have offered a good solution to a certain point; however, viscosities over 10 to 20 centipoise (cP) a challenge for most standard spring-based autoinjectors systems, and can cause serious issues with completeness of injections. Whilst many companies are looking at adapting spring-based delivery systems for higher viscosities, there are some drawbacks to this mechanism. The kickback produced when the injection discharges can be uncomfortable, even frightening to patients in some instances. This could potentially deter the patient from using the device as often as is required; discomfort can have a negative impact on compliance.
The high impact of spring-based delivery can also lead to breakage of the primary drug container in certain situations. Most prefilled syringes were never designed to be used with autoinjectors, and as such, industry has seen issues with breakage prior to injection and during injection. Other issues include poor siliconization leading to stalling or incomplete injections, flange strength is often insufficient, and the rigid needle shield can prove incompatible with the grabber/removers of some devices.
Another challenge is the volume of a dose. The volumes used in autoinjectors routinely fall between 0.2 to 1.2 ml. However, to address many of the aforementioned viscosity issues, and to facilitate larger volume injections, the volumes now required are moving well beyond this level. To this end, 2.25-ml autoinjectors are now entering the market. As volumes increase well beyond 0.2 to 1.2 ml, the time required to administer also increases. For 1-ml injections, a target time of less than 10 seconds is considered the norm for autoinjectors. This threshold exists because patients have difficulty holding a device against their skin for longer than 10 seconds. There is no standard threshold for volumes of 2 ml at this time; however, it still stands that beyond 10 seconds patient compliance is potentially affected.
When designing autoinjectors, device companies must also take into account that patients are expected to use autoinjectors by themselves at home, with no supervision from medical professionals. This has a potential impact on both patient safety and compliancy, as one bad experience with a device or medication in general can be a barrier to future use. As a result, autoinjectors are given to patients as part of a package of tools that train patients in their use or support patients in training themselves. This will often include a teaching session with a nurse or practitioner, image-based protocols, as well as video tutorials. Trainer devices that allow the patient to practice the action of injection without the presence of a needle are also available. Additional training is sometimes provided through home visits by nurses hired by the pharmaceutical company. This has worked particularly well in the area of multiple sclerosis.
LATEST INNOVATIONS
 Clearly new mechanisms are needed to improve the experience for patients and contribute to enhanced compliance and adherence. Several companies are looking at new and disruptive technologies to find a solution, such as unique spring configurations or electromechanical processes. With autoinjectors in high demand by the biopharma industry, gas-powered delivery systems may have the solution to many of the difficulties the industry is facing.
Clearly new mechanisms are needed to improve the experience for patients and contribute to enhanced compliance and adherence. Several companies are looking at new and disruptive technologies to find a solution, such as unique spring configurations or electromechanical processes. With autoinjectors in high demand by the biopharma industry, gas-powered delivery systems may have the solution to many of the difficulties the industry is facing.
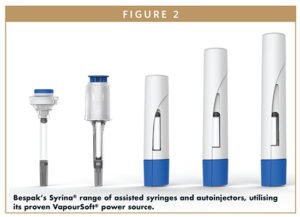
To meet the demands for suitable autoinjectors, Bespak has developed a novel container of liquefied gas that provides sufficient energy, as pressurized vapor, to power delivery of the drug. The container is essentially a miniaturized form of the gas canisters used in inhaler devices, which the company has been producing for more than 50 years. The adaptation, known as VapourSoft®, provides a smooth, dampened delivery action that reduces injection impact seen with current spring-based mechanisms.
BENEFITS OF GAS-POWERED DELIVERY
Gas-powered delivery has the advantage of being incredibly flexible. Due to the variety of liquefied gases available, it’s possible to provide the complete spectrum of pressure ranges within a single container format. This allows a single device system to manage a variety of delivery options, including different viscosities (up to 300 cP and much higher with a new variation on the technology), injection volumes, and primary containers. Its size means that it can also be readily incorporated into different types of delivery systems, such as autoinjectors, wearables, and bolus systems.
A smooth delivery profile also makes gas-powered delivery suitable for glass primary containers, for which breakage has historically been a serious issue, resulting in a number of recalls.5Issues with breakage and incomplete delivery are exacerbated when using viscous solutions as more force is required to power delivery. Because gas-powered delivery has a soft start and low actuation force, this technology has no impact on the glass syringe and thus can be used in combination safely even with high-viscosity solutions, without fear for contamination or loss of part of the dose.
Being able to provide a constant delivery profile in all situations is vital. Such a delivery system is able to deliver a smooth, consistent delivery profile, vital for patient comfort, completely avoiding any recoil, often seen in spring-based systems. Consequently, this smooth delivery profile also ensures that the full contents of the syringe are dispensed regardless of initial fill level.
BEYOND THE DELIVERY PROFILE
Patient compliance is known to be influenced by the size and shape of the delivery device. A compact and versatile format allows gas-powered delivery technology to be used in unique, non-linear form factors, enabling ergonomic designs and making it an ideal choice for devices that need to be highly customized for specific patient groups.
For patient groups required to carry a device on their person, the size of the device can influence compliance. A compact device will more readily fit into a pocket, and so becomes more convenient to carry.
Ease-of-use has become a much bigger consideration with the rise of self-administration. In indications such as arthritis in which movement can be difficult or painful, ease-of-use becomes even more important, and flexibility of device design becomes key. Device manufacturers work with human factors experts to find out what type, shape, or form of device is preferred by patients.
The space required in a device for a gas-powered delivery system is significantly reduced compared to other options. The compact nature of the technology and additional space afforded by the removal of a plunger rod allows for very unique configurations of the device. These are driven by the drug and the target patient group and the preference of the pharma company. Although some companies do choose to go with standard device designs, many prefer to have a unique shell for the device, allowing for features that improve grip dexterity and other aids to use, in addition to providing brand differentiation.
One trend we continue to see is the use of the two-step autoinjector, which is placed uncapped against the injection site, and triggered by a simple push-on-skin action. In addition to providing greater flexibility with regard to the size and shape of the final device, a compact delivery mechanism also allows the inclusion of additional features to further enhance compliance, such as connectivity.
WHAT’S NEXT FOR SELF-ADMINISTERED DEVICES?
The ability to include additional features, such as connectivity capabilities into patient-acceptable devices is already in demand. An increased emphasis on smart devices is also predicted, with data used to improve adherence by increasing transparency between the doctor and patient and making complying with the correct dosing regimen easier for the patient. In general, “Smart Health” has become a hot topic in the industry, and is set to revolutionize patient-care. Used in conjunction with autoinjectors, such technology will enable healthcare professionals to track where, when, and how medication is used by each patient. This will provide invaluable insight into an individual’s usage history, including dose regime compliance. For allergy sufferers, such data could help identify potential triggers, whilst for those with a chronic condition this information could inform personalized dose regime modifications.
Throughout the next several years, injectable devices will have to continually evolve to keep pace with developments in biologics. Dosing is likely to become less frequent still, and this will likely have an impact on the volumes needing to be administered. Upward of 2 ml, wearable delivery devices will likely provide the solution. Wearables are able to offer more flexibility around volumes and timing of delivery, enabling low-dose, long-term injection – delivering the drug slowly over a long period of time, or even delivering the drug 24 hours after fitting the device for convenience.
A compact gas-powered delivery approach, based on proven long-established technologies, is ideal for delivering the challenging biotherapeutic formulations in the most demanding situations. As new injection mechanisms emerge, their success will depend on a balance of factors; size of the technology and therefore real-estate required in the device, smoothness of delivery profile, as well as cost. Importantly, to supply the breadth of different devices needed by today’s industry, device companies need to be able to provide a choice of delivery mechanisms to customers.
REFERENCES
- Autoinjectors Market: Rising Incidence of Anaphylaxis Attack Among the Adult Population Expected to Elevate the Demand for Autoinjectors: Global Industry Analysis and Opportunity Assessment 2016-2026 –http://www.futuremarketinsights.com/reports/auto-injectors-market.
- Injectable Drug Delivery Market worth 624.50 Billion USD by 2021 –http://www.marketsandmarkets.com/PressReleases/injectable-drug-delivery.asp.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3964020/.
- http://www.pmlive.com/pharma_intelligence/oral_biologics_delivery_still_elusive_908436.
- http://www.contractpharma.com/issues/2012-01/view_features/the-glassquandary
To view this issue and all back issues online, please visit www.drug-dev.com.

Steven R. Kaufman is Global Business Development Lead, Bespak Europe Ltd, where he is responsible for the global business development of injectable devices, such as auto-injectors and wearable injection systems. He has more than 13 years of experience in bringing drug delivery devices to market and forming strategic alliances with related suppliers and consultants. He works actively with the Bespak Innovation team, which designs and develops advanced drug delivery devices, and is part of the Senior Leadership Team. He earned his IMBA in Marketing and International Business from the National Chengchi University.
Total Page Views: 5614









