GALECTIN-DIRECTED THERAPIES - Targeting Galectin-3 Protein in Drug Development
INTRODUCTION
Fibrogenesis is a major cause of morbidity and mortality, and anti-fibrotic agents have been termed a holy grail of drug development. The galectin-3 (gal-3) protein appears to be critical to the process of fibrogenesis, and targeting gal-3 could potentially treat a broad spectrum of diseases.
Two approaches have been taken to develop drugs that bind gal-3. One involves modified disaccharides (TD139), and the other uses large polysaccharides that contain galactose (GR-MD-02). Studies are being conducted on the use of GR-MD-02 in non-alcoholic steatohepatitis (NASH), chronic inflammatory skin diseases, including plaque psoriasis and atopic dermatitis, and in combination cancer immunotherapy.
WHY GALECTIN-3 IS AN ATTRACTIVE DRUG TARGET
The galectin-3 (gal-3) protein is an intriguing new drug target to treat a variety of human disorders, a number of which represent large unmet medical needs. Gal-3 is a member of a family of lectin proteins that binds to galactose-containing glycoproteins. First discovered as a major expressed protein in macrophages, gal-3 is expressed in many cell types in the body but predominantly in immune cells. Expression of gal-3 is increased in many chronic inflammatory and fibrotic diseases, as well as in multiple types of cancer cells. Because the immune system is involved in many diseases, the targeting of gal-3 has a broad spectrum of potential disease targets, including organ fibrosis (ie, liver, lung, and kidney), skin disease, ocular disease, atherosclerosis, heart failure and arrhythmia, and diabetes. In heart failure, for example, levels of serum gal-3 correlate with poor prognosis.
Starting in 2006, interest in gal-3 significantly increased based on experiments in gal-3 null mice, which are otherwise normal but do not express the gal-3 protein. These gal-3 null mice, when insulted in various ways, were found to be resistant to the development of fibrosis in multiple organs, including liver, lung, kidney, and heart. These data suggest that gal-3 is critical to the process of fibrogenesis, a major cause of morbidity and mortality in patients and one of the most important unmet medical needs. In fact, anti-fibrotic agents have been termed a holy grail of drug development with multiple pharma and biotech companies seeking new drugs.
DRUGS TARGETING GALECTINS
Two approaches have been taken to develop drugs that bind gal-3: Modified disaccharides (TD139; Galecto Biotech), and large polysaccharides that contain galactose (GR-MD-02; Galectin Therapeutics). Both approaches yield molecules that are not well absorbed orally and must be given parenterally, although TD139 is being delivered by the inhaled route in a Phase I study in IPF (idiopathic pulmonary fibrosis). Galectin Therapeutics has taken the approach of using a modified, naturally occurring carbohydrate polymer that contains chains of galactose as a drug that binds to gal-3 (GR-MD-02).
There are significant differences in the binding properties of these two drugs, with TD139 having higher affinity for the carbohydrate recognition domain of gal-3 but GR-MD-02 having a binding stoichiometry of ~5 molecules of gal-3 per drug molecule and binding to a broader area of amino acids in gal-3. While it is unknown how these differences may affect ultimate effectiveness, it is notable that both molecules had a similar effect in a mouse model of lung fibrosis.
CLINICAL STUDIES UTILIZING GR-MD-02
GR-MD-02 is being used currently in three areas of development: 1) chronic inflammation and fibrosis in non-alcoholic steatohepatitis (NASH); 2) chronic inflammatory severe skin diseases, including plaque psoriasis and atopic dermatitis, and; 3) combination immunotherapy for cancer. While the major value driver for GR-MD-02 is as a NASH drug, there are early clinical results showing efficacy in severe skin disease.
Liver Fibrosis & NASH
Preclinical results show that GR-MD-02 has significant anti-fibrotic effects in multiple models, including liver (fatty liver and toxin induced), kidney, lung, pulmonary artery, and heart fibrosis. NASH was chosen for development of GR-MD-02 because it is one of the most common liver diseases, with 1 in 4 individuals in the world having fatty liver and about 2% of those destined to die of complications of NASH cirrhosis. There are currently no approved drugs for NASH. NASH is recognized as one of the largest potential markets for drug development today, with the global market in 2025 predicted to be as large as $40 billion.
NASH is a chronic disorder with fat accumulation in the liver resulting in inflammation, cell death, and progressive fibrosis leading over decades to the end stage of scarring, or cirrhosis. Gal-3 is markedly upregulated in liver disease and has effects on the two main liver cell types involved in fibrogenesis: macrophages and stellate cells (Figure 1). Inhibition of gal-3 may reduce the fibrogenic myofibroblasts, reduce macrophage recruitment and activation, and potentially increase the macrophage activity to reduce collagen and fibrosis.
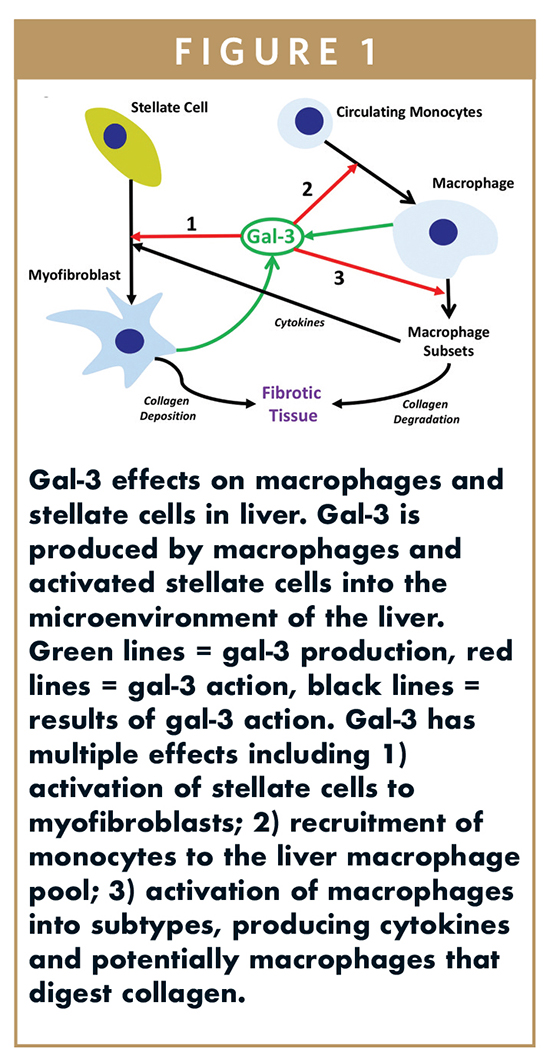
GR-MD-02 has been shown in mouse models of NASH to reduce fat, inflammation, and cell death and to both prevent and reverse fibrosis (Figure 2A and 2B).1 In this mouse model of NASH, gal-3 is markedly increased in macrophages (Figure 2C), and this is significantly reduced with GR-MD-02 treatment (Figure 2D). Additionally, in a toxic model of rat cirrhosis, GR-MD-02 has been shown to reverse fibrosis and cirrhosis (Figure 2E and 2F), despite continuation of the toxic insult, and partially reverse the portal hypertension associated with cirrhosis.2 Portal hypertension is the primary reason for complications in humans with NASH cirrhosis and a potentially acceptable regulatory endpoint in clinical trials.
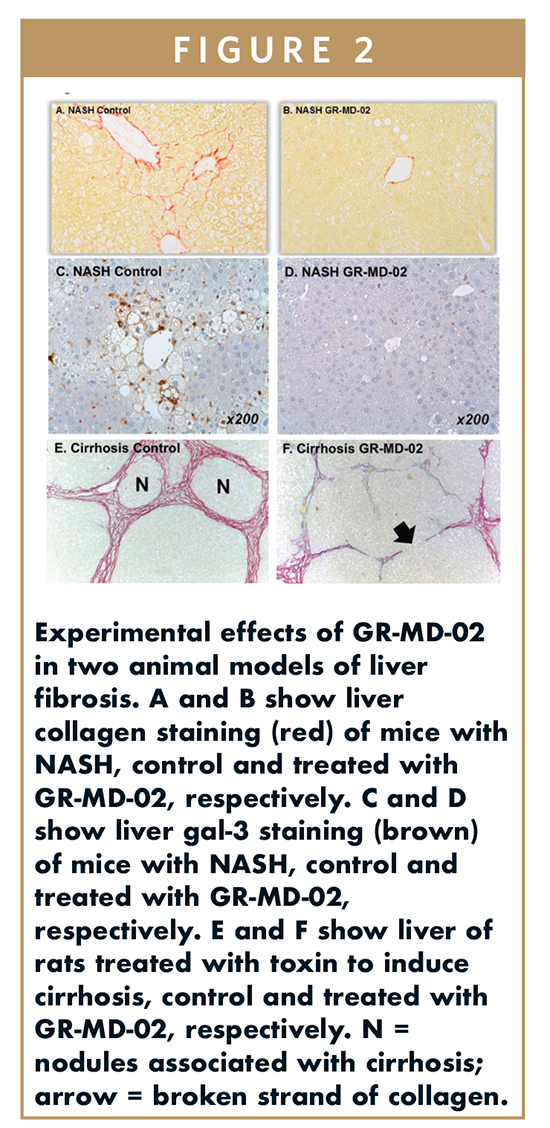
Based on these robust preclinical findings, GR-MD-02 therapy in development is being directed at patients with NASH cirrhosis, with the goal of reducing portal pressure by reducing fibrosis and thereby improving outcomes. After obtaining an IND for NASH with advanced fibrosis and fast-track designation, two Phase I trials and an exploratory Phase II trial were completed showing good safety and a lack of drug interactions. This led to the currently underway Phase IIb clinical trial in patients with NASH cirrhosis and portal hypertension (NASH-CX).
There are a number of important elements in the NASH-CX trial. First, it is one of only three currently ongoing clinical trials in NASH cirrhosis, and the next one to read out top line data in this calendar year. The trial has enrolled 162 patients in three treatment arms, placebo and two doses of drug, with a treatment duration of 52 weeks. The primary endpoint of the trial is the baseline adjusted reduction in portal pressure as assessed by hepatic venous pressure gradient (HVPG), which is directly related to patient outcomes and is potentially an acceptable regulatory endpoint for provisional approval with follow up outcomes data. Additionally, secondary endpoints will evaluate change in liver biopsy, serum biomarkers, complications, and several non-invasive measures of liver structure and function including FibroScan (Echosens) and 13C methacetin breath test (Exalenz). The study is powered at >80% to demonstrate a difference in HVPG of at least 2 mmHg, a change which is potentially clinically significant in these patients. More than 25% of subjects have completed the trial, and it is on track to report top line data in December 2017.
The NASH-CX trial is a significant milestone in NASH therapy as well as a proof-of-concept for therapies directed at gal-3. Most of the many current ongoing clinical trials in NASH are directed to therapy in pre-cirrhotic NASH, but the NASHCX trial is the next trial to read out in the more advanced patients with NASH cirrhosis. Success in this area could be a breakthrough finding for liver cirrhosis and additionally open the possibilities for gal-3 targeted therapy in fibrotic disorders of other organs.
Clinically Meaningful Effect of GR-MD-02 in Psoriasis & Atopic Dermatitis
Serendipity struck the GR-MD-02 clinical development program when a patient enrolled in a Phase I NASH trial had a remarkable remission of her psoriasis, a skin disease not known to resolve itself naturally. In addition to this clinical finding, research publications suggested the potential importance of galectin-3 in psoriasis.
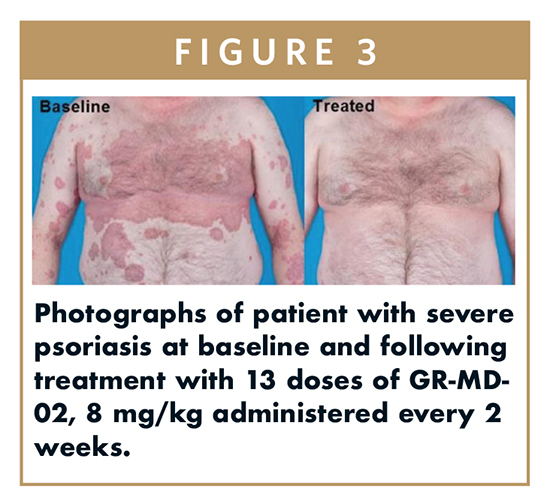
On this basis, an exploratory, openlabel, Phase IIa trial was conducted in five adult patients with moderate-to-severe plaque psoriasis [PASI (Psoriasis Area and Severity Index) ≥ 12 and BSA (Body Surface Area) ≥ 10%]. One patient had an 80% reduction in PASI 30 days after their last infusion (13th) (Figure 3), while the other four patients reached 50% reduction in PASI by their 10th infusion.
Scientific studies also show that there is a link between the galectin-3 protein and disease activity in atopic dermatitis with increased amounts in the skin of patients and reduced disease in mice with atopic dermatitis that lack the galectin-3 protein.
GR-MD-02 treatment of severe and refractory atopic dermatitis was also studied in three patients in an open label, investigator-initiated study. All three patients showed clinical response as determined by reduction of the Eczema Area and Severity Index (EASI) score at week 12 having received six every-other-week doses, with two patients achieving a 64% and 74% reduction in EASI, respectively, at 6 weeks after receiving only three doses of GR-MD-02.
These early clinical results demonstrate activity of GR-MD-02 in two severe skin diseases, providing some confidence that there may be clinical activity in other gal-3 dependent disorders. Importantly, there is a higher incidence of psoriasis in patients with NASH. While significantly more development work and formulation will be required to demonstrate clinical utility, these findings open up an entirely new target area for severe skin diseases, with potential market utility in the relatively underserved area of moderate-to-severe atopic dermatitis.
Cancer Immunotherapy
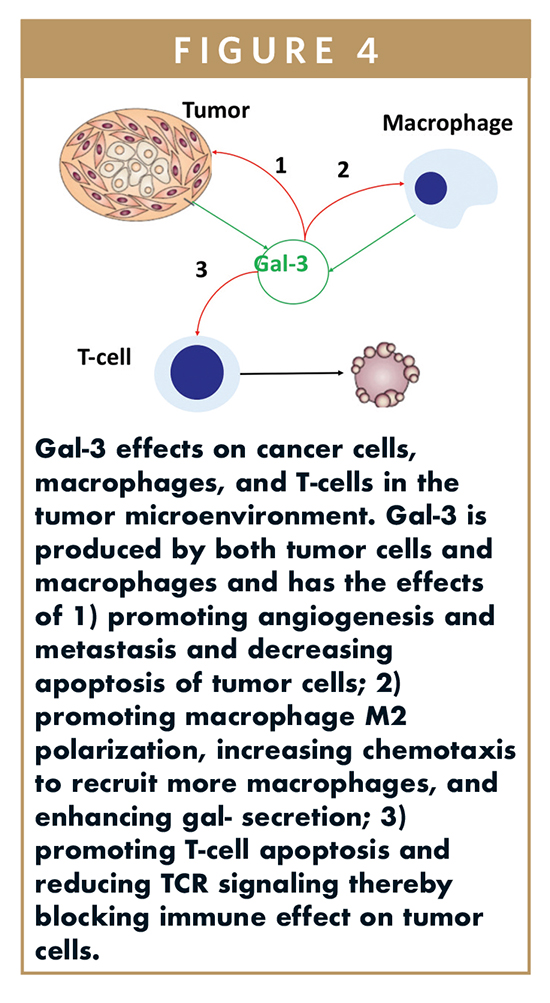
Gal-3 expression is increased in most cancers and secreted into the tumor microenvironment. There are multiple effects of gal-3 in cancer (Figure 4), including the promotion of angiogenesis and metastasis and decreased apoptosis of tumor cells, macrophage M2 polarization, increased chemotaxis to recruit more macrophages, and enhanced macrophage gal-3 secretion, and T-cell apoptosis and impairment of TCR signaling reducing the ability of immune system to kill tumor cells.
The Providence Cancer Center (Portland, OR), recently reported early results of GR-MD-02 combined with pembrolizumab (KEYTRUDA®) in patients with advanced melanoma, oral/head and neck cancer (OHN), and non-small cell lung cancer (NSCLC). Six subjects, five with advanced melanoma, were enrolled in the lowest dose cohort (2 mg/kg GR-MD-02) with one partial response and one mixed response in the five melanoma patients. Figure 5 is a chest CT scan of the patient with a partial response showing a marked reduction in tumor size at week 12 of therapy, after three doses of combined GR-MD-02 and pembrolizumab.
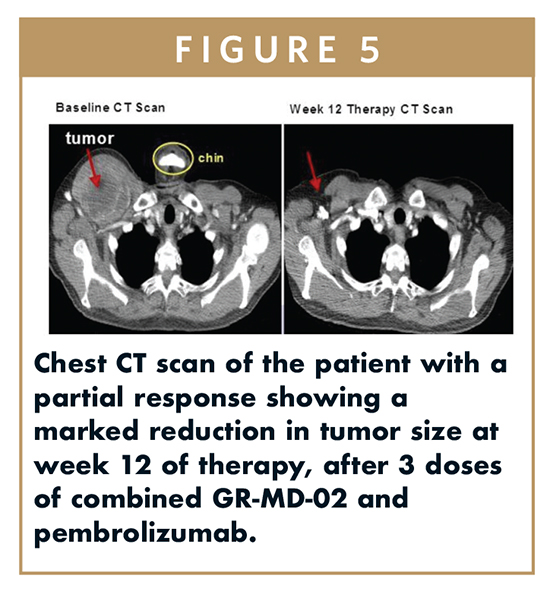
The investigators were encouraged by these early safety results, and although one cannot conclude whether the one partial response was related to GR-MD-02, the response provides a clinically relevant signal to follow as GR-MD-02 doses are escalated. This study is ongoing, and a decision to progress to Phase II will be based on the response rate of the combination of pembrolizumab with GR-MD-02 as compared to historical response rates to pembrolizumab alone.
GR-MD-02 HAS A STRONG SAFETY PROFILE
In each of the clinical trials with GRMD-02, the drug has shown to be safe and well tolerated, with no serious adverse events ascribed to the drug. At this time, the total number of doses of GR-MD-02 administered to humans is nearly 3000. This is encouraging for the development program, given the high rate of drugs that are dropped from development based on significant toxicities. Additionally, given that GR-MD-02 is a complex carbohydrate, it is metabolized via different mechanisms than typical small molecule drugs, and a lower likelihood of toxicities based on drug metabolites is anticipated.
THE FUTURE OF GALECTIN DRUG DEVELOPMENT
The treatment effect of GR-MD-02 on psoriasis and atopic dermatitis provides evidence for efficacy of one anti-gal-3 drug in human disease. Ongoing trials in NASH cirrhosis, with results at the end of 2017, and combination chemotherapy will add to the data. Additionally, presentation of results are awaited from the Phase I trial using TD139 in the treatment of IPF. Each of these trials should add to the foundation of information on the potential for anti-gal-3 therapeutic approaches, which could extend to multiple important diseases.
We are only at the beginning of understanding gal-3 targeted therapy and the full potential of this approach. The future likely holds additional high affinity, specific, galectin inhibitors that are bioavailable by routes other than the two currently in development, parenteral and inhaled. In this regard, Galecto Biotech has suggested it has oral inhibitors in preclinical development, and Galectin has a discovery program for identification of small molecule inhibitors of gal-3. Such oral inhibitors will likely open many possibilities for galectin-directed therapies for a broad range of human disease.
REFERENCES
1. Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One. 2013;8(12):e83481.
2. Traber PG, Chou H, Zomer E, Hong F, Klyosov A, Fiel MI, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS One. 2013;8(10):e75361.
To view this issue and all back issues online, please visit www.drug-dev.com.

Dr. Peter G. Traber is President, CEO, and CMO of Galectin Therapeutics and President Emeritus of Baylor College of Medicine, where he was CEO from 2003 to 2008. From 2000 to 2003, he was Senior Vice President of Clinical Development and Medical Affairs and CMO of GlaxoSmithKline plc. He served as CEO of the University of Pennsylvania Health System and was Chair of the Department of Internal Medicine and Chief of Gastroenterology for the University of Pennsylvania School of Medicine. Dr. Traber has also managed a molecular biology research laboratory and published over 100 articles of original research, reviews, and book chapters. He earned his MD from Wayne State School of Medicine, his BS in Chemical Engineering from the University of Michigan, and a certificate in medical leadership from Wharton Business School.
Total Page Views: 11500









