Issue:January/February 2017
FORMULATION DEVELOPMENT - Optimizing the Spray-Drying Parameters for a Formulation of Nanoparticles-In-Microparticles System of Acetazolamide
ABSTRACT
In the current research, a spray-dried nanoparticles-in-microparticles system (NiMS) of Acetazolamide (ACZ) was formulated. The objective behind the research was to examine the consequence of spray-drying parameters that are inlet temperature and feed rpm on the entrapment efficiency, loading capacity, percentage yield, and particle size on formulating NiMS of ACZ. The prepared NiMS were evaluated for entrapment efficiency, loading capacity, percentage yield, and particle size, and it was found that PP-5 (formulated using an inlet temperature of 160°C and feed rpm 30) has a maximum entrapment efficiency 17.94% (w/w), loading capacity 33.2% (w/w), percentage yield 25.8% (w/w), and smallest particle size of 763 nm out of all the five formulations (PP-1 to PP-5). The DSC analysis of PP-5 suggested that the entrapment of the nano and microparticles and spray-drying generate a noticeable crystallinity of ACZ and confers a nearly amorphous state to this drug. Infrared analysis of PP-5 showed no interaction between drug and polymer during the formulation process. The SEM of PP-5 found that the particles are of irregular shape, typically in the range of 2.5 to 3.5 μm. Therefore, NiMS of ACZ have been successfully formulated and it was observed there was an effect of inlet temperature and feed rpm of the spray dryer on the entrapment efficiency, loading capacity, percentage yield and particle size. Thus it can be concluded this study can be beneficial for the formulation of NiMS of ACZ by spray drying.
INTRODUCTION
In recent years, significant consideration has been focused on the expansion of novel drug delivery systems (NDDS). The reasons behind the development of NDDS include drug delivery to the site of action without any significant immunogenicity reactions, biological inactivation, or the potential side effect to the critical tissues, such as liver, lungs, bone marrow, kidney, etc. The main goal in developing NDDS is to advance the therapeutic efficacy and safety of existing drugs by altering the biodistribution pattern of the drugs, reducing the amount and frequency of dosing.1 NiMS are the systems that reduce drug dosage frequency and increase patient com- pliance.2 The most important purpose in designing NiMS is to control particle size, surface properties, and make pharmacologically active agents release to achieve site-specific drug action at the dosage regimen and therapeutically optimal rate.3 Chitosan has excellent potential in designing nanoparticulate drug delivery, the ability to control drug release, and is biocompatible with living tissue. Chitosan is a natural carbohydrate polymer and prepared by the partial N-deacetylation of chitin. Chitosan-based NiMS have advantages, mainly for the design of novel nanoparticulate drug delivery systems, due to their desirable properties such as biodegradability, bio- and mucoadhesivity, biocompatibility, and hydrophilic character that facilitate the poorly absorbable drugs administration across the various epithelial barriers, such as intestinal, nasal, and corneal mucosa.4 Acetazolamide (ACZ) is a carbonic anhydrase inhibitor. In the eye, carbonic anhydrase inhibition decreases the flow of sodium, bicarbonate, and water into the posterior chamber. Suppression of this reaction in the ciliary process reduces the aqueous humour production by almost total enzyme inhibition. Thus, the intraocular pressure in both normal and glaucomatous eyes is reduced.5
Spray drying is a technique described as the feed conversion into a dry particulate form by subjecting the feed into a hot drying medium from a fluid state. Liquids of various types, such as slurries, emulsions, and dispersions, can be converted into solid particles with preferred size, porosity, shape, density, and distribution.6 There are various spray-drying benefits, which include control of size and shape and porosity and density, a rapid and simple process that produces free flowing particles, scalability and reproducibility, cost effectiveness, and enhanced dissolution rate of drugs.7 The spray-drying process effectiveness is exaggerated by different factors, such as inlet temperature, outlet temperature, flow rate, and feed concentration and rate.8 Therefore, it is desirable to evaluate the various effects of spray-dried parameters on the ACZ-loaded NiMS. In the present study, the goal was to formulate NiMS of ACZ by spray drying, and the objective behind the study was to investigate the effect of spray-drying parameters, i.e., inlet temperature and feed rpm on the percentage yield, entrapment efficiency, loading capacity, and particle size.
MATERIALS
Chitosan (95% deacetylated), having a molecular weight of 40 to 80 kDa, was purchased from Fluka Chemika, Switzerland. Sodium tripolyphosphate (NaTPP) and glacial acetic acid were purchased from Central Drug House, New Delhi. ACZ was obtained from Kaizen Pharmaceuticals, Chandigarh. Tetrabutylammonium hydrogen sulphate was obtained from Spectrochem Pvt. Ltd, Mumbai. Acetonitrile (HPLC grade) was purchased from Rankem, New Delhi.
METHODS
Preparation of ACZ-Loaded NiMS
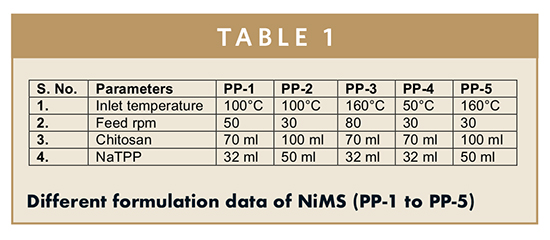
In the current research, NiMS were prepared using the ionic gelation method.9 In this method, chitosan (0.5% w/v) was dissolved in 0.3% v/v glacial acetic acid. 500 mg of the ACZ was dissolved in it. The solution of sodium tripolyphosphate (NaTPP) (0.2% w/v) was prepared in distilled water. The NaTPP solution (32 to 50 ml) was added to the chitosan solution (70 to 100 ml) dropwise stirring continuously. The suspension was spray dried (JISL, Navi Mumbai) at a feed rate (30, 50, and 80 rpm) and at specified temperature (50°C, 100°C, and 160°C) to get the free flowing powder. The aspirator blower capacity was 118 Nm3/hr, and the nozzle size was 0.7 mm with auto de-blocking device. To study the effect of various formulation variables, NiMS were prepared as shown in Table 1.
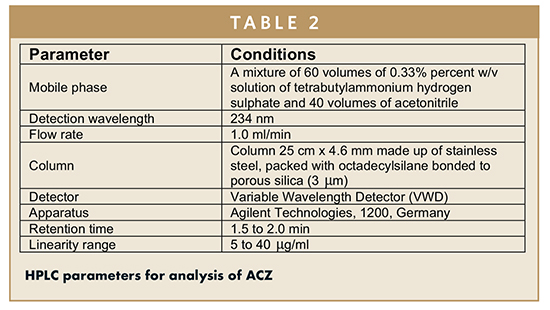
In the current research, using Agilent Technologies 1200 series, Germany [Quaternary pump, a stainless steel column 25 cm x 4.6 mm, packed with octadecylsilane bonded to porous silica (3 μm), UV detector dual wavelength]. HPLC analysis was performed utilizing the parameter as shown in Table 2.10
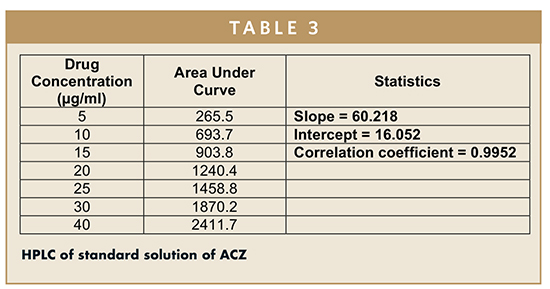
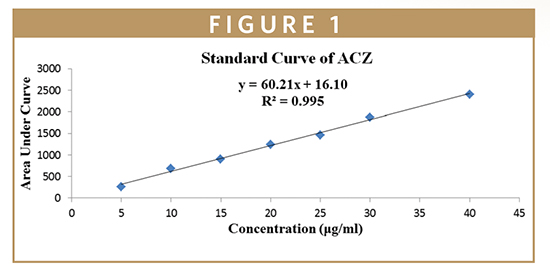
Initially, the column was washed with a mixture of acetonitrile and methanol with varying flow rate for half an hour and decreasing ratio of methanol. After that, the column was saturated with 1.0 ml/min of flow rate for 30 mins and mobile phase. After that, the standard drug dilution samples (5 μl) were injected and run for 10 min. The drug retention time was found between 1.5 to 2.0 min. In Table 3, HPLC of standard solution of ACZ is shown, and the calibration curve was prepared as shown in Figure 1.
CHARACTERIZATION OF NIMS
Particle Size Analysis
For analysis of particle size, using Zetasizer Instrument (Beckman Coulter Desla Nano, USA) equipped with the hydro-dispersing unit by dissolving 2 mg of sample in 5 ml of distilled water, the dilution of sample was analyzed. In a polystyrene cuvette in hydro-dispensing unit, the dilution of sample was filled, and the scan was carried out at 64 runs per sample. At the end of scan, the average diameter of all the 64 runs were taken out and recorded as Z-average.
Percentage Yield, Entrapment Efficiency & Loading Capacity
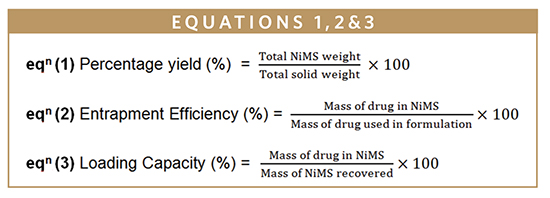
The ACZ percentage yield, entrapment efficiency, and loading capacity were determined directly using NiMS. The analyses of HPLC were carried out using a system (Agilent Technologies 1200 series, Germany) that consisted of a column 25 cm x 4.6 mm made up of stainless steel packed with octadecylsilane bonded to porous silica (3 μm). The mobile phase was a mixture of 60 volumes of a 0.33 % w/v solution of tetrabutylammonium hydrogen sulphate and 40 volumes of acetonitrile. The wavelength was set at 234 nm, and the flow rate was 1.0 mL/ min.10 The percentage yield, entrapment efficiency, and drug loading were calculated (Equations 1, 2, & 3).11
Differential Scanning Calorimetry (DSC) Analysis
DSC was carried out on DSC Q10 (Waters Corporation, USA) using indium as standard to calibrate the instrument. In sealed pans of aluminum, samples were heated at a rate of 10°C/min under nitrogen atmosphere (60 ml/min) and temperature range from 30°C to 300°C, with empty pan as reference.
Fourier Transform Infrared (FTIR) Spectrophotometry
FT-IR is a technique of obtaining infrared spectra using an interferometer by first collecting an interferogram of a sample signal, and then obtaining the spec trum performing a Fourirer transform on the interferogram. Fourier transform IR spectra were recorded on FT-IR (Alpha, Bruker, Germany). Over the range of 500 to 3500 cm-1, the spectra were recorded.
Scanning Electron Microscopy (SEM)
The NiMS were mounted on metal stubs using double-sided tape and using Sputter gold coater and visualized under scanning electron microscope. The particles were coated with gold to a thickness of about 450 Å.12 The surface appearance and morphology of spray-dried PP-5 was observed via SEM (Hitachi S 3400 N, Japan).
RESULTS & DISCUSSION
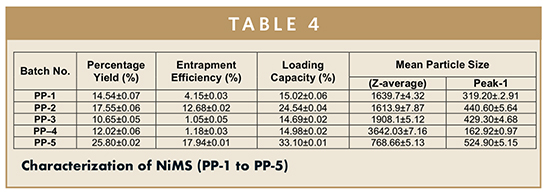
In the current research, the spray-drying technique was used for the conversion of suspensions into solid particles (nanoparticles/microparticles) and no additional drying adjuvant was needed. TPP is an anion that can form cross-linkage involving ionic interactions between the TPP molecules (negatively charged) and amino group (positively charged) of chitosan. The opalescence indicated the development of particles with a size range of nanoparticles to microparticles with the incorporation of ion TPP to chitosan solution.13 In spray drying, there are various factors that affect the formulated product, such as inlet temperature, outlet temperature, feed rpm, feed concentration, aspirator rate, nozzle size, etc. Out of these factors, inlet temperature and feed rpm of spray dryer were selected to evaluate, and the analysis of percentage yield, entrapment efficiency, loading capacity, and particle size of all five formulations were carried out and are tabulated in Table 4. It has been observed that the PP-5 formulations have the highest percentage yield (25.8%) with highest entrapment efficiency (17.94%), highest loading capacity (33.2%), and with a smallest particle size of 763 nm.
Particle Size Analysis
By means of laser diffractometry, using Zetasizer instrument (Beckman Coulter Desla Nano, USA) equipped with a hydro-dispersing unit, particle sizing experiments were carried out. The particles size analysis of each batch (PP-1 to PP-5) have been tabulated in Table 4.
Percentage Yield, Entrapment Efficiency & Loading Capacity
When we compared the results of percentage yield, entrapment efficiency, and loading capacity of PP-1 and PP-2, which have the same inlet temperature, PP-1 showed the better result as compared to the PP-2, due to the PP-2 having the lower feed rate so that the solution gets efficient time to get converted into solid particles. While the entrapment efficiency, loading capacity, percentage yield of two formulations (PP-3 and PP-4) were lowest because the prepared solution was not completely converted into the solid particles due to the higher feed rate and lower temperature, respectively. The best results showed by the PP-5 with respect of having highest percentage yield, entrapment efficiency, and loading capacity due to the higher inlet temperature and lower feed rpm of spray dryer. The results of percentage yield, entrapment efficiency, and drug loading of all five batches (PP-1 to PP-5) are tabulated in Table 4.
Differential Scanning Calorimetry (DSC) Analysis
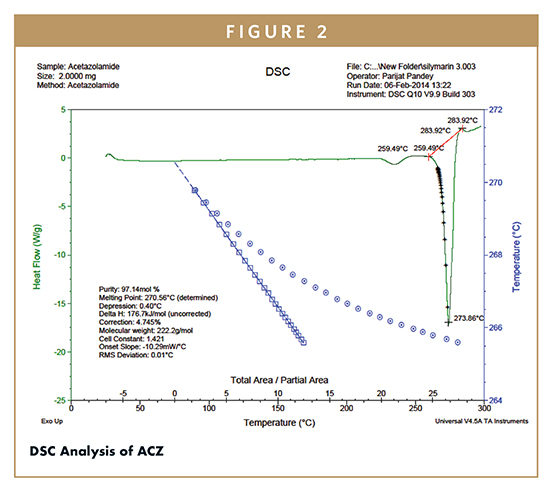
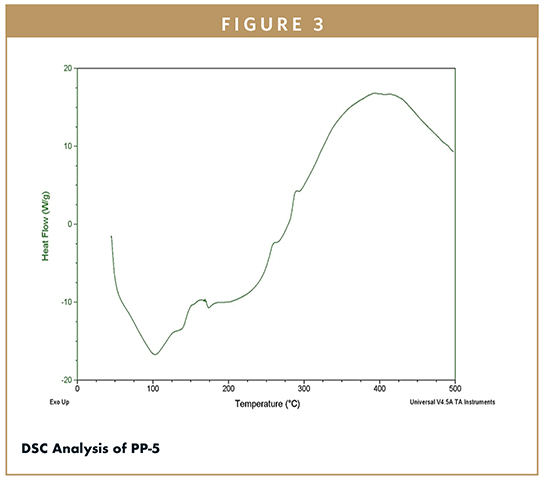
The ACZ was confirmed by DSC analysis, and there was a sharp peak at 273.86°C almost corresponding to its melting point (260.52°C) (Figure 2). During NiMS formation, the loss of endothermic peak of PP-5 (Figure 3) showed that the drug may have been dispersed in the amorphous form in the polymer matrix.11,14 The entire amalgamation of the drug in the NiMS formulation indicates the molecular dispersion of the drug is within the system. By spray drying, the peak of drug disappeared in NiMS, indicating the drug was molecularly dispersed in the medium of chitosan as a solid solution. These outcomes suggest the spray-drying process produces a marked decline in crystallinity of ACZ and confer to this drug a nearly amorphous state.11
Fourier Transform Infrared (FTIR) Spectrophotometry
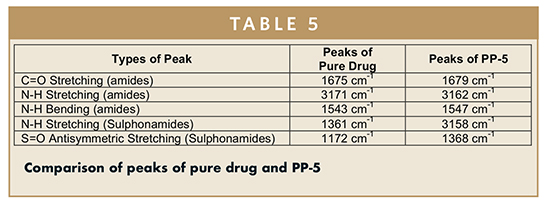
The spectra were recorded for pure drug (ACZ) and ACZ-loaded PP-5 and the comparison of peaks of pure drug and PP-5 are tabulated in Table 5. There was no major variation in the FTIR spectra of pure ACZ and PP-5. No significant shifting of functional peaks, no overlapping of characteristic peaks, and also no appearance of new peaks were observed upon comparison of obtained spectra with reference spectra. The results suggest drug stability during the entrapment process. The FTIR data suggested that molecular interactions that could alter the chemical structure of the drug did not occur. Therefore, no chemical interface between the functional group of drug and polymer exist.14
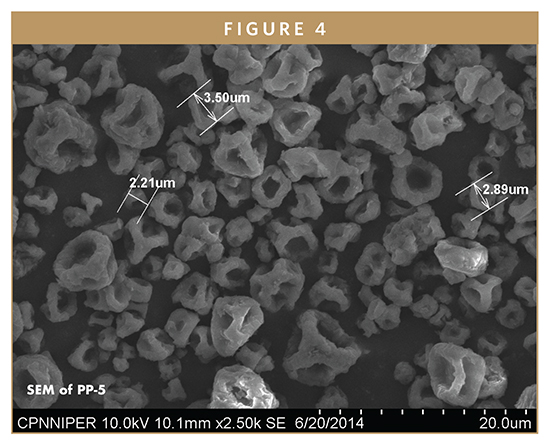
Scanning Electron Microscopy (SEM)
Figure 4 shows the SEM of the PP-5 of spray-dried powder. It was found that the particles were of irregular shapes, typically in the range of 2.5 to 3.5 μm. The irregular particles may be attributed to the fact that during the spray-drying process, high pump rates result in a large volume of spraying solution to be dried, but heated air might not transform liquid droplets into solid droplets, immediately leading to the formation of bigger irregular-shape particles that are not completely dried and tend to form aggregates.15
CONCLUSION
Spray drying has been successfully applied to prepare ACZ-loaded NiMS. The effect of spray-drying parameters that are inlet temperature and feed rpm of the spray dryer were evaluated on the percentage yield, entrapment efficiency, loading capacity, and particle size of ACZ-loaded NiMS. The PP-5 was regarded as the best batch because it has the highest entrapment efficiency (17.94%), loading capacity (33.2%), and percentage yield (25.8%) with the smallest particle size (763 nm). The current research supports the approach to prepare redispersible ACZ-loaded NiMS in a powdered form by using the spray-drying technique and also marked the effect of spray-drying parameters on the formulation of ACZ-loaded NiMS. From the research, it has been concluded that the formulation with higher inlet temperature and lower feed rpm resulted in maximum percentage yield along with entrapment efficiency, loading capacity, and smallest particle size.
REFERENCES
1. Bhowmik D, Chiranjib R, Jayakar RM. Role of nanotechnology in novel drug delivery system. J Pharm Sci Tech. 2009;1(1):20-35.
2. Pandey P, Dureja H. Nanoparticles-in-microparticles systems (NiMS) a an overview. J Innovative Biol. 2014;1(4):195-199.
3. Lee YS, Johnson PJ, Robbsins PT, Bridson RH. Production of nanoparticles-in-microparticles by a double emulsion method. a comprehensive study. Eur J Pharm Biopharm. 2013;83:168-173.
4. Patil MS, Bhoskar M. Optimization and evaluation of spray-dried chitosan nanoparticles containing doxorubicin. Int J Curr Pharm Res. 2014;6(2):7-15.
5. Leaf DE, Goldfarb, DS. Mechanism of action of acetazolamide in the prophylaxis and treatment of acute mountain sickness. J Appl Physiol. 2007;102:1313-1322.
6. Sollohub K, Cai K. Spray drying technique: II. current applications in pharmaceutical technology. J Pharm Sci. 2009;99(2):587-597.
7. Kaushik D, Dureja H. Recent patents and patented technology platforms for pharmaceutical taste masking. Recent Pat Drug Deliv Formul. 2014;8(1):37-45.
8. Kaushik D, Dureja H. Taste masking of bitter pharmaceuticals by spray-drying technique. J Chem Pharm Res. 2015;7(4):950-956.
9. Pandey P, Dureja H. Spray-dried nanoparticles-in-microparticles system (NiMS) of acetazolamide using central composite design. Nanotech Nanosci Asia. 2016;6(in press).
10. Indian Pharmacopoeia. Government of India, Ministry of Health and Family Welfare. The Indian Pharmacopoeia Commission, Ghaziabad. 2014;985-990.
11. Elzoghby AO, Samy, WM, Elgindy NA. Novel spray-dried genipin-crosslinked case in nanoparticles for prolonged release of a alfuzosin hydrochloride. Pharm Res. 2012;30:512-522.
12. Kommanaboyina B, Rhodes, CT. Trends in stability testing with emphasis on stability during distribution and storage. Drug Dev Ind Pharm. 1999;25(7):857-868.
13. Li FQ, Yan C, Bi J, Lv WL, Ji RR, Chen X, Su JC, Hu JH. A novel spray-dried nanoparticles-in-microparticles system for formulating scopolamine hydrobromide into orally disintegrating tablets. Int J Nanomed. 2011;6:897-904.
14. Pirooznia N, Hasannia S, Lotfi AS, Ghanei M. Encapsulation of Alpha-1 antitrypsin in PLGA nanoparticles: In vitro characterization as an effective aerosol formulation in pulmonary diseases. J Nano Biotech. 2012;10(20):1-15.
15. Wan LSC, Heng PWS, Chia CGH. Preparation of coated particles using a spray-drying process with an aqueous system. Int J Pharm. 1991;77:183-191.
To view this issue and all back issues online, please visit www.drug-dev.com.

Dr. Harish Dureja earned his Master’s degree from Punjabi University, Patiala, and his Doctorate (Gold-Medal) from M.D. University, Rohtak. He is presently working as Associate Professor in the Department of Pharmaceutical Sciences, Maharshi Dayanand University, Rohtak (India). He has 16 years of experience in teaching and Research. He has written 10 invited book chapters/monographs in various books published by international publishers. He is author of more than 115 publications in various national and international journals of repute. He has been awarded two research projects for research on anti-cancer drug delivery by the AICTE and UGC. His current research interests include in silico ADME modelling, nanoparticulate drug delivery, and pharmaceutical regulatory affairs.

Mr. Parijat Pandey earned his Master’s degree in Industrial Pharmacy and is presently pursuing his PhD as Research Scholar (JRF) in the Department of Pharmaceutical Sciences, M.D. University, Rohtak. He has published 8 manuscripts in various international and national journals and is a life member of IPGA.
Total Page Views: 8326









