Issue:April 2014
DISPOSABLE TECHNOLOGY - Use of Disposable Technology in Clinical Fill & Finish Manufacturing: Benefits & Considerations
INTRODUCTION
Use of disposable technology – from pump systems to filling needles – is gaining traction in the clinical manufacturing of injectable drugs. Traditional stainless-steel equipment is expensive, takes a long time to procure, and requires lengthy qualification/cleaning validation. But successful use of disposables doesn’t simply mean swapping out stainless-steel parts for plastic. The following will review not only the benefits of using disposables, but the real-world variables to consider when converting to single-use technology. The pathway begins with the question, why use disposables?
CLEANING VALIDATION OUT; MORE API IN
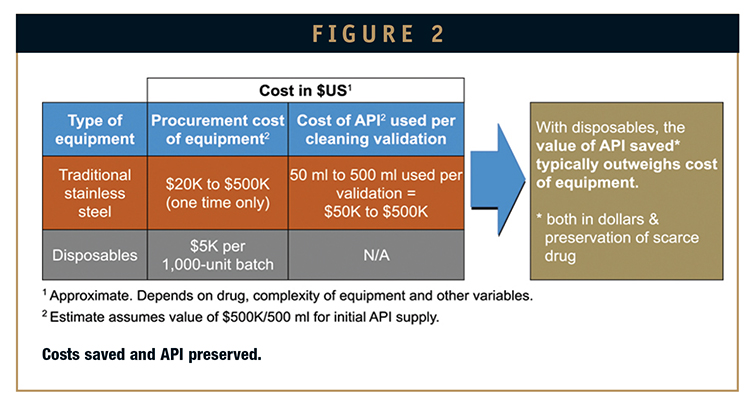
One of the clear benefits to disposables is that their use eliminates the need for cleaning validation. Use them once; throw them out. Poof – 1 to 3 months is cut instantly from the timeline to first batch (Figure 1).
Once clinical manufacturing is underway, disposables also eliminate the additional 1 to 3 months per year, approximately, required to requalify the cleaning procedure and equipment.
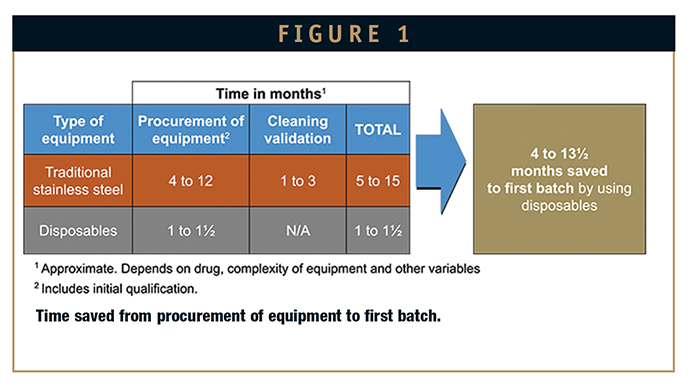
But perhaps even more important, precious API need no longer be “wasted” on cleaning validation studies. Five hundred milliliters of API – the amount that would fill a small soda bottle – can be valued at $500,000 or more. Moreover, that may be all the drug a company has produced in the early stages of development, following years of work. In larger-scale traditional compounding equipment, two-thirds of that initial API may be consumed in a single cleaning validation run. So for many companies, disposables are not merely nice to have; they can be a must-have in order to be able to proceed with drug development.
QUICKER TO GET, LESS EXPENSIVE TO BUY
It’s easy to spend $100,000 for tanks, needles, and other components of traditional, dedicated fill-and-finish equipment. Some complex custom systems can cost as much as a half-million dollars.
That’s too much for companies, especially small ones, to invest before even knowing their drug will work. They also need product in hand within 4 months to be competitive and maintain economic viability. This timeline simply is not possible with stainless steel. Off-the-shelf models generally take 4 to 5 months to procure, and custom equipment up to 1 year, including qualification.
Conversely, single-use systems can be procured in 4 to 6 weeks and cost around $5,000 per 1,000-unit batch. The cost of disposables can add up with increasing numbers and sizes of batches. But because cleaning validation is eliminated, the savings in API almost always eclipse equipment costs (Figure 2).
PROVIDES FLEXIBILITY DURING A TIME OF UNCERTAINTY
During the early stages of development, knowledge is still limited about the drug product and processes. Adjustments are inevitable. With traditional equipment, the moment a formulation is changed, for example, that $50,000 dedicated stainless steel compounder that took 9 months to procure will instantaneously turn into a hunk of useless metal.
Disposable equipment enables quick and inexpensive last-minute “tweaking” – or wholesale changes. It’s also highly economical when scaling up batch sizes from 100 units to 10,000 to 100,000, compared to purchasing dedicated equipment for each increase.
CREATING A COMPREHENSIVE DISPOSABLE PATHWAY
Citing the benefits of disposables is easy. But making them a reality is harder and requires careful planning. Many companies have already incorporated disposable elements within their clinical manufacturing operations, but few have created a facility-wide disposable pathway. The general principle is that whenever an ingredient or solution is in direct contact with equipment or components, use disposables. That applies to activities involving weighing API and excipients, material preparation and assembly, compounding, filtration, and filling operations. Single-use technology is not required for equipment that is in indirect contact with the drug, such as lyophilizers or RABS gloves.
CRITERIA FOR SELECTING SINGLE-USE TECHNOLOGY
The disposables market is already large and growing. Where to begin? Here are some considerations when choosing a supplier to meet your needs:
• What kind of validation data package does the supplier have readily available?
– Have they checked material compatibility data?
– What is the material’s chemical resistance?
– What is its leachables and extractables profile?
– What is its shelf-life?
– How easily can I access their quality documentation (extremely important)?
• Can the supplier work within your lead times?
• Are they willing to customize their platforms to fit your needs, when necessary?
• Do they have a solid track record in the bio/pharma industry?

DELVING DEEPER INTO PRODUCTS
Once you locate a potential supplier, drill down on the technology itself. This is just a sampling of the type of questions to ask:
• Does the available equipment meet your specification requirements?
• Can materials be autoclaved (if applicable)?
• Do mixing systems cover a wide range of applications, including both slow and fast mixing (applicable to a platform technology)?
• What is bag quality? More certified features carry a higher price; lower standards carry risk of leaking. Risk mitigation requires a well-considered balancing act.
• Can parts and components be connected aseptically?
• Are components easy to mount, exchange, and disassemble within a RABS system (if applicable)?
• If components come in bulk, are they compatible with your cleaning procedures?
Don’t stop at questions. Before committing, obtain samples from several suppliers and conduct trials to determine if their wares are truly compatible with your own operations and up to your quality standards. You may find that a mix of suppliers best serves your needs, depending on their various strengths and weaknesses.
THE REAL-WORLD HURDLES OF DISPOSABLES: TWO CASE STUDIES
Theory is fabulous. Then there’s real life. While the disposables market is growing fast, it’s still in its infancy. Before making a commitment, conduct extensive examinations of both materials and suppliers to verify that both meet your requirements. Be ready for surprises, be patient, and above all, be vigilant. Here are two real-world tales of problems that one company encountered and what it did to solve them.
MATERIALS TESTING
Test, test, and test again before selecting single-use equipment because the field of disposables is still so new. A company that was trying to establish a site-wide disposable pathway was in search of an acceptable disposable filling needle. The first supplier provided a product that did not comply with established cleaning procedure. The glue failed. The second supplier’s needle discolored with cleaning and did not properly seal to an adjoining component. As an intermediate solution, the company discarded stainless-steel needles following each batch, in keeping with the facility’s single-use framework. Finally, the company found a third supplier that was able to provide high-quality disposable filling needles.
SUPPLY CHAIN & DOCUMENTATION
The disposables supply chain can be complex. One of the suppliers the company had selected started subcontracting as it grew. Eventually, one subcontractor supplied manufacturing and parts assembly; a second, packaging; and a third, gamma radiation. Each subcontractor supplied its own quality documentation, which was in order, but the three were not compiled together as a single, complete package. The company worked closely with the supplier to tighten processes so that all documentation was properly packaged to fully comply with regulatory requirements.
These are just two of the hurdles the company encountered, but they were not unexpected. The world of disposables is evolving. Be ready to work as partners with your suppliers, while continuing to scan for new options and improvements in technologies.
DISPOSABLES ARE NOT A PANACEA
Although they offer a clear technological advantage, don’t count on disposables alone to drive success in clinical manufacturing. For example, using filling equipment with short tubing lengths to minimize line losses is critical to preserving API during early stage manufacture. And knowing how to develop the right processes during development for a smooth transfer to commercial manufacturing can cut many months off time-to-market.
Disposables, however, are already playing a vital role in the acceleration of clinical manufacturing. That can mean greater return on investment for life sciences companies, but more important, faster delivery of new therapies to the patients who need them.
To view this issue and all back issues online, please visit www.drug-dev.com.

Dr. Claudia Roth was appointed President of Vetter Development Services USA, Inc. in 2010. She is responsible for the operations and administration of Vetter’s early stage clinical manufacturing facility in Chicago, IL, the company’s first US site. Dr. Roth joined Vetter, a leading contract development and manufacturing organization (CDMO), in 2000 at the company’s headquarters in Ravensburg, Germany. Starting in the company’s aseptic production group, she later moved to Vetter’s research and development organization, where she built and led the division’s first process development and implementation function. Dr. Roth studied chemical/process engineering at the Friedrich-Alexander University of Erlangen in Nuremberg, Germany, earning her PhD in Lyophilization, which she executed at Roche Diagnostics in Mannheim, Germany. A recognized expert in lyophilization, she regularly presents at industry forums on topics related to aseptic manufacturing.
Total Page Views: 4170