Issue:March 2015
COMBINATION PRODUCTS - Human Factors & Combination Products
INTRODUCTION
Manufacturers of combination products are increasingly being asked by regulators to perform human factors (HF) testing alongside the clinical trials program. This comes as a surprise to many pharmaceutical companies, in particular those who are developing their first combination product. As an active HF practitioner who works with combination products globally, I see first-hand the confusion caused when manufacturers of combination products are suddenly asked by regulators to present a report of their HF data. Often, this is because the client has realized very late in the product development cycle that HF studies are likely to be required. But there are some very clear principles to follow, and in this review article, I will discuss the most important considerations when setting out to perform HF studies with combination products.
WHAT ARE COMBINATION PRODUCTS?
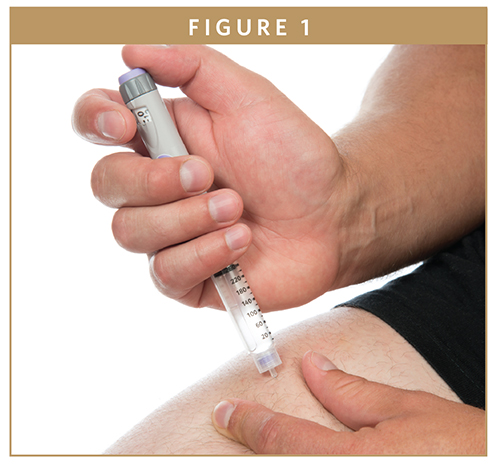
Combination products sit in a grey area between medical devices and pharmaceutical products, with features of both product types. The US Food and Drug Administration (US FDA) defines combination products as “diagnostic or therapeutic products that combine drugs, devices, and/or biological products.” This means that some type of technology (such as a hand-held injection device) is used to deliver a measured amount of drug from a container through an exit port and onto the administration site. This dual aspect to combination products is one reason why regulators often try to address both the clinical and HF programs with the same review team. The most common types of combination products are pulmonary inhalers (eg, dry powders or pressurized aerosols), injection systems (eg, pen injectors and auto-injectors), and infusion systems (eg, ambulatory syringe pumps delivering continuous subcutaneous drug delivery). Other types include nasal sprays, creams, eye drops, and ear drops.
COMBINATION PRODUCTS & HUMAN FACTORS
The development of combination products is gathering pace, with many of the world’s pharmaceutical companies developing them as a key part of their new product pipeline. In many cases, firms are dusting off their older drugs and generating new intellectual property around a new delivery technology. New injection technologies, smaller infusion pumps, and smarter inhalation systems are all being developed.
Regulators are catching up, and there is a growing demand on manufacturers to provide evidence that their new technology can be used safely. The FDA in particular is increasingly proactive and is currently updating its guidance to manufacturers on human factors (HF), with combination products very much in their sights.
SO WHAT’S THE PROBLEM?
The science of drug testing is well developed, and there are clear and comprehensive guidelines on how to construct clinical studies, and a well-established body of “best practices” available to industry and researchers alike. There is also a reasonable amount of knowledge (and guidance) on how to perform HF testing for medical devices; however, the science of HF testing for combination products is less well developed, and there is, as yet, no specific guidance from regulators.
Because combination products have only recently become center stage, there is still a lack of clarity about how HF testing should be applied to them. In my experience, there is also a lack of consistency from regulators when they review HF data for combination products.
A common complaint is that regulators seem to be applying an unduly stringent risk-focused approach, even when the drug involved has been used for many years, and also when the product type is widely used. For example, one organization I know has been asked to provide evidence to mitigate use-related risks anew for their auto-injector, even though it is similar to devices that have been available for some time. This is despite the fact there is widespread experience of their use amongst healthcare professionals, and auto-injectors are not generally regarded as high risk devices.
Another complaint we hear often is that there is conflicting guidance from different reviewers within the same regulatory body. For example, one reviewer might request an untrained group to be included in a study, whilst a different reviewer might say that an untrained group is not required.
HOW DOES THE USER EXPERIENCE DIFFER?
The users’ experience with a combination product is fundamentally different to traditional oral drug products, such as tablets and capsules. With oral drug products, the drug is presented as a tablet, capsule, or a liquid; often the user needs only perform a very few tasks to administer the drug either to a patient or to themselves, such as opening the packaging, removing a tablet, or pouring a liquid onto a spoon and then swallowing. However, with combination products, the user must master many more of the device’s components. This may mean performing 8, 10, 12, or more tasks successfully, such as assembling it, inserting a drug cassette, switching it on, reading the dose counter, priming an infusion line, checking for dose delivery, stopping delivery, switching off, and disassembling. A failure to perform any one of these tasks correctly could mean that the patient does not receive the intended dose, or receives an overdose or some other harm.
WHAT DO REGULATORS WANT?
Regulators require evidence that your combination product can be used safely and effectively by its intended users. As with other medical devices, their focus is three-fold; the intended users, the use environments, and the user interface.
So they will be looking for evidence of safe and effective use of your product from these three perspectives. FDA guidance states that medical devices should be “adequately safe and effective for its intended users to use it for its intended use in its intended use environment(s).”1 In practice, this means providing data in particular from your summative HF testing program, and involves showing the performance outcomes of study participants during simulated use. Specifically, you will need to show that the user interface of your combination product (such as the instructions, labels, dose counters, etc) can guide your product’s intended users toward safe use, especially for those tasks that are critical.
ARE YOU EXPECTED TO REMOVE ALL RISK OF HARM?
There is a common belief that regulators expect manufacturers to remove all risk of use-related harm. In our experience, this is not the case; the regulators do understand there may be limits to what you can realistically do with the current state of technology, although they definitely do expect you to demonstrate that you have quantified the risks and done everything you can reasonably do to reduce it.
Thus, you may also need to prepare a justification as to why you do not intend to try to further remove risk. The FDA guidance clearly states that you may provide reasons why you are not intending to continue development; in other words, you may be justified in stating that:
1. Further modifications to the user interface (remember this includes the device itself, plus instructions, patient leaflets, packaging, and labelling) would not further reduce risk. AND
2. Further modifications are not feasible or practicable given the current state of the technology (eg, when there is no technology that can anticipate a user’s next action, or where there is no way to remove the risk of a user running out of medication during a weekend). AND
3. The residual risks are outweighed by the benefits that would accrue to users; this may be particularly the case for novel technologies that address previously unmet clinical needs.
Whether you can use the argument that you cannot afford further changes is debateable; a financial argument alone is unlikely to be accepted, but one that is combined with an explanation as to why further changes are not feasible or practicable given your resources and the state of technology, then that is likelier to be acceptable.
GENERATING THE RIGHT HF DATA FOR COMBINATION PRODUCTS
Human factors specialists approach a combination product in the same way they would do for a medical device. First, by defining some of the basics, such as:
Intended Use: What is the intended use of your combination product? Examples might be “the continuous subcutaneous delivery of drug X for patients with condition Y whilst being mobile.”
Intended Users: Who are the product’s intended users, and what is known about them? Examples might be “adults over 18 years in the United States with moderate-to- severe rheumatoid arthritis who are currently using a disease-modifying drug by injection” (remember, you will need to be very specific, for example, about age, sex, ethnicity, and any other characteristic that may influence usability).
Use Scenarios: What use scenarios are expected to be most frequently encountered when the product is on the market, and what are the reasonably foreseeable worst-case scenarios? Examples might include “using an inhaler at home during the night in low light conditions whilst laying down in bed” (again, you need to be specific).
Use-Related Risks: What risks of harm are posed by the use of your combination product, and are these clearly documented? These need to link directly to your use-related risk assessment, and you will need to make sure that you are testing at the very least all of the higher risk tasks.
GETTING THE TESTING RIGHT
There are some critical questions to ask as you plan your human factors testing program:
Getting the Tasks Right: Are you testing the right tasks? You should be testing those tasks that are associated with use-related risks. But in combination products, you have the additional risks associated with the drug. If the risks are not related to the intended user using the device component, then it is probably not necessary to test them. However, if your risk assessment includes any drug-related risks that are potentially caused by a user handling/using/applying the device component (eg, by controlling the dose delivered), then it should be evaluated.
Test the Right Users: Are you testing the right users? The intended users of combination products may include a healthcare professional (HCP) who teaches the patient how to use it, and may also configure the product. For example, with ambulatory infusion pumps, a healthcare professional may configure the bolus volume and the flow rate. Users may also include a pharmacist who might teach patients how to use their inhalers. Also, with the increasingly elderly and frail patients, there may be a caregiver (such as a partner, child, or friend) who may need to perform some or all of the tasks.
WHAT HF DATA IS REQUIRED FOR COMBINATION PRODUCTS?
In principle, the HF data requirements for combination products are similar to those for medical devices with a few important differences, as follows:
Device or Drug?: Your combination product is designed to deliver a drug safely and effectively. Your focus should be on the use-related risks, rather than the drug-related risks. In other words, your HF program is not intended to provide evidence that the drug itself is safe. Your focus is on the safety and efficacy of the technology. So start with a use-related risk assessment and make sure it focuses on the technology, not the drug. Remember, you are not retesting the drug, you are gathering evidence that users can deliver the drug safely and effectively.
Home Use: Many combination products are intended for use at home. It is possible (or indeed likely) that some users will have received no training. They may rely on your instruction leaflet, or they may need to ask a friend or partner for help. This places additional burden on you to show that the instructions can help an untrained or inexperienced user to use it safely.
Multiple Reviewers: As we have already mentioned, there is a range of experience among regulatory reviewers with regard to HF. Because a combination product involves a drug, it is entirely possible that your HF data gets reviewed by a drug regulator who may have little experience of reviewing HF data. Whilst some reviewers may ask for help from a colleague with HF expertise, you may get asked questions that appear to have little relevance to best HF practices. In practice, this means that you will have to help the regulator by working hard to provide a clear, concise report with suitable references, and a scientific justification for your arguments, in particular, with regard to any residual use-related risks.
Device-User Interface: What elements constitute the interface between the combination product and the user? This is where there is likely to be the most difference between oral pharmaceuticals and combination products, because there will be multiple elements to the user interface. Examples may include a dose counter, a carrying case, a charging port, or perhaps an app. There may be disposable items, such as a needle, and there may be peripheral items, such as a spacer device for aerosol inhalers. Your HF focus is on the interface between your combination product and its intended user, so you will need to gather data on any aspect of the user interface that supports safe use. Your combination product may require the use of disposable items, such as an infusion line, a disposable mouthpiece, or a disposable needle. It may include a carrying case with a window that enables users to view the remaining dose to be delivered. These constitute a system, and whilst you are not expected to prove that someone else’s product is safe, you will be expected to test the whole system, including peripheral items.
A FEW FINAL COMMENTS
Manufacturers of combination products are being asked to provide rigorous data on the safety of their product when its intended users use it. These challenges are not going to diminish, but with a focused approach and some sound HF principles, manufacturers can navigate the challenges successfully.
Disclaimer: The opinions expressed in this article are based on the author’s experience only and cannot necessarily be taken as specific recommendations.
To view this issue and all back issues online, please visit www.drug-dev.com.

Richard Featherstone is an experienced human factors and usability practitioner and is joint Founder of Medical Device Usability in the UK, where he specializes in combination products. He has 30 years of experience in the pharmaceutical and human factors industries and works globally. He is regarded as an expert on the usability of inhalation technologies, and designs and runs human factors testing programs globally, with extensive experience of summative testing in the US for combination products, including inhalers, injection systems, and infusion devices. MDU is a human factors and usability consultancy that focuses exclusively on medical technologies, such as medical devices and combination products. Based in Cambridge UK, MDU performs human factors and usability testing globally, including extensive experience in the US. To contact Mr. Featherstone, email him at richard@medical-device-usability.com. To contact MDU by phone, call +44 1223 214 044.
Total Page Views: 8393