Issue:November/December 2014
ABUSE-DETERRENT MARKET - What’s in the Pipeline? Abuse-Deterrent Products
INTRODUCTION
Reducing misuse and abuse is one of the more interesting applications of formulation and drug delivery technology to real-world challenges. Problems related to the abuse of central nervous system-acting products are well known and yet remain a significant challenge for the medical community, families, as well as law enforcement and the courts.
Serious attempts to reduce the abuse of prescription drugs, most notably opioids, have been ongoing for more than a decade with some significant advances realized. Yet there remains much to be done. From the perspective of the pharmaceutical industry, there are at least two approaches that promise at least some relief for this problem. The ideal solution from the perspective of many is the development of increasingly well-tolerated novel agents capable of treating indications such as pain, anxiety, depression, and hyperactivity, without any abuse liability. This “holy grail” solution is yet to be realized. The fallback approach has been to look to the pharmaceutical sciences for ways to reduce the abuse and misuse of agents that have a long history of efficacy and safety when used as prescribed. We will use the term “abuse-deterrent” to describe these desired features and benefits. This expression encompasses a wide variety of actions all related to reducing the non-prescribed use of a product, in terms of intent or procedure.
We decided it would be interesting to interrogate the PharmaCircle database and see what successes have been achieved with respect to abuse-deterrent formulations in terms of approved and pipeline products. We also took a more general look at the formulation approaches being applied to abuse deterrence.
Some background; this short review includes only products and technologies that have reached the stage of clinical development. Research and preclinical products, and their associated technologies, have not been included. For the purpose of this article, some products that have not reported results or activity for 4 or more years have been labeled as inactive, even though the sponsoring companies may still list them in their product pipelines.
THE ABUSE-DETERRENT PRODUCT PIPELINE – APPROVED & CLINICAL-STAGE PRODUCTS
Querying the PharmaCircle Products & Pipeline database with the terms “abuse-deterrent” and “abuse-resistant” returned a total of 129 products as being at some stage of announced development, marketed through clinical to preclinical and research. Limiting this list to products that are active and either approved, filed with regulatory bodies, or in clinical development reduces the number to a much more manageable list of 53 products. This excludes an additional 10 clinical products that have been formally discontinued or have provided such limited updates to suggest they are in effect discontinued. This list is current as of mid-September 2014.
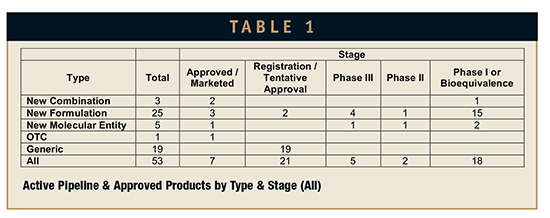
The distribution of these products by type and stage are summarized in Table 1. What is a little bit surprising is the number of generic abuse-deterrent products (19) that have received tentative approval or have their applications under review. The four approved products being targeted by these generics are Shire’s Vyvanse, Endo’s Opana ER Crush-Resistant, Acura’s Oxecta, and Purdue Pharma’s reformulated OxyContin.
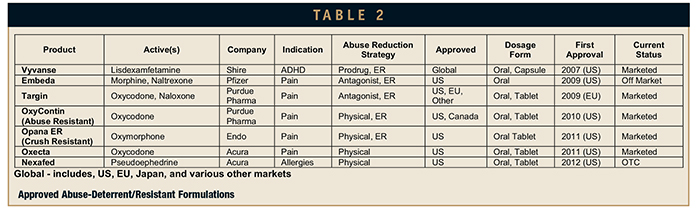
If we take out the generics, there remain a total of nine products that are Marketed, Approved, or in Registration. A total of seven products have been approved with a direct or implied abuse-deterrent or abuse-resistant claim. These products are listed in Table 2.
FORMULATION STRATEGIES
Four general formulation strategies are currently employed to reduce or eliminate the potential for the abuse and misuse of psychoactive pharmaceuticals. These are:
Type 1- Formulations that physically limit the ability of products to be mechanically or chemically modified for the purpose of injection, insufflation, or rapid oral absorption.
Type 2 – Formulations that include an antagonist or aversive agent that blocks the desired properties of the product when abused, or makes it unpalatable or toxic when repurposed for administration by injection or insufflation.
Type 3 – Modified formulation-release products that limit the possibility of rapid absorption of the active. This can be achieved through some sort of molecular modification (prodrug) or sustained-release engineering not easily overcome using mechanical, physical, or kitchen chemistry procedures.
Type 4 – The fourth approach combines two or more of the aforementioned approaches, most commonly some sort of modified-release combined with physical/chemical-resistance features. The subcategories are:
Type 4a: Type 1 & Type 2
Type 4b: Type 1 & Type 3
Type 4c: Type 2 & Type 3
Type 4d: Type 1 & Type 2 & Type 3
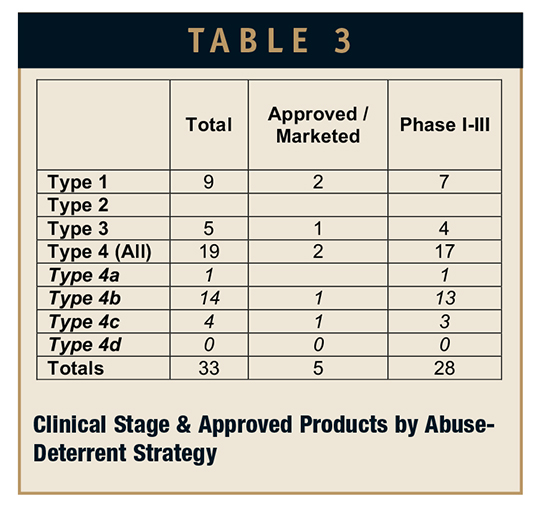
An analysis of the approved and clinical development pipeline as a function of technology approach is presented in Table 3. (Note: generic products are not included in this analysis.) Some assumptions were made regarding the exact abuse strategies of certain pipeline products of which there was limited public information. A little bit of explanation is in order to address what appear to be inconsistencies with the figures in Table 3.
There are no “pure” Type 2 products in development as best as can be determined. The Type 2 strategy, incorporating an antagonist or aversive agent, is only seen in combination with some sort of modified/extended-release technology. Of course, extended-release, Type 3 was the original abuse-deterrent strategy until it was realized that these products could be crushed, overcoming the modified-release characteristics of the products and negating any abuse-prevention benefits. One immediate-release development program using an aversive agent, niacin, as opposed to an antagonist, was terminated after an FDA Advisory Panel suggested this approach introduced safety issues.
At this point, there are no approved or clinical-stage products identified as being Type 4d, incorporating all three abuse-deterrent strategies, although there appear to be a couple in the preclinical stage.
The sharp-eyed reader will note that there is a one-product discrepancy between Tables 1 and 3. This is an outlier product, a transdermal formulation of fentanyl that makes claim to reducing the potential for abuse by exhausting the fentanyl through efficient delivery and leaving a negligible amount of active in the patch after the prescribed 3-day dosing. This makes the “used” patches less attractive for “smoking” or extraction using kitchen chemistry techniques.
REGULATORY CONSIDERATIONS & GENERIC FORMULATIONS
It’s remarkable to see how many 505(j) products are lined up at the US FDA waiting to capture the generic opportunity represented by currently approved abuse-deterrent products. The leading product from a units and revenue perspective, Purdue Pharma’s OxyContin, is likely to be subject to generics as soon as October 2014 on the basis of a settlement with Actavis. This agreement limits the number of units that Actavis will be permitted to distribute after the FDA approves their generic.
The whole question of the regulatory requirements necessary to secure a label claim of abuse-resistant or deterrent is still not clear. Purdue managed to secure language in its US product labeling for OxyContin that reviewed the abuse-deterrence studies conducted for the product. This contrasts with the new formulation of Opana ER from Endo that is identified as crush-resistant, but carries no abuse-resistant or deterrent information in its package insert. Clearly, there is a minimal amount of data that needs to be provided to secure abuse-resistant and/or deterrent language in the product labeling. Exactly what this might be is not obvious to the casual observer.
This issue of exactly what performance targets and studies are required to capture an abuse-deterrent claim will have an impact on the approval and claims of future products, including generics. Regardless, products that include any sort of abuse-resistant or deterrent features are a net benefit to the public whether or not they receive the corresponding claims from the regulatory bodies.
ABUSE-DETERRENT FORMULATIONS – THE FUTURE
Although still in relative infancy, the whole area of abuse-deterring formulations is likely to grow up very quickly, and likely without any privilege. It’s not unlike the mid-1990s, where sustained-release formulations quickly became a standard part of every company’s formulation toolbox. It was at this point no longer necessary to secure external expertise to create a long-acting formulation of a proprietary molecule. This point was emphasized by the parallel emergence of sustained-release generic products in the mid to late 1990s.
It may be that the golden age of abuse-deterrent formulation technologies has already passed before it has had a chance to flourish. That’s not to suggest abuse-resistant and deterrent formulations in the pipeline won’t be approved and provide important therapeutic benefits. Rather, the opportunity to profit through market share and pricing flexibility as a result of any significant technology or regulatory exclusivity will be limited. The actives, for the most part multi-source opioids and stimulants, provide no real patent exclusivity. And with the development of multiple abuse-deterrent formulation strategies, there appears to be little potential for any true exclusivity from a technology perspective. It is difficult to imagine that any company will be able to capture the type of profit with abuse-deterrent products or technologies as has been enjoyed by Purdue Pharma and their reformulated OxyContin.
It’s not hard to predict that we will see more and more abuse-resistant and deterrent formulations of opioids and stimulants hit the market in the near future, followed by their generic equivalents. The real money to be made will be found in those products that change the whole paradigm – novel molecules that retain desired analgesic or psychoactive properties but without any inherent addictive or abuse-reinforcing properties. It’s possible these molecules will be discovered, but it does not seem it will be anytime soon. In the meantime, patients, physicians, and society as a whole will need to look to the ingenuity of pharmaceutical science professionals to provide meaningful near-term solutions.
To view this issue and all back issues online, please visit www.drug-dev.com.

Dr. Josef Bossart is Managing Director of The Pharmanumbers Group, a boutique research and consulting group providing the biopharmaceutical industry with analysis and insights that improve business outcomes. He has more than 3 decades of experience in the biopharmaceutical sector, including senior sales, marketing, business development, and management positions within Big Pharma, Specialty Pharma, and Emerging Pharma companies. He earned his PhD in Medicinal Chemistry from The Ohio State University, College of Pharmacy.

Dr. Tugrul T. Kararli earned his PhD in Pharmacology from the University of Florida and his MBA from DePaul University. Dr. Kararli worked at Searle/Pharmacia for 18 years and held various positions and responsibilities within the Pharmaceutical Sciences department, participating in pharmaceutics, product development, and drug delivery activities. As the Chairman of the Global Drug Delivery Technology Team at Pharmacia, he was responsible for identifying, planning, and executing the drug delivery technology strategies for marketed and development products. Dr. Kararli has authored numerous articles on various aspects of pharmaceutics and drug delivery and holds more than a dozen US and international patents. Currently, he is the Founder and President of PharmaCircle LLC, a knowledge management service company in the drug delivery and pharmaceutical/biotechnology fields.

Mr. Kurt Sedo is Vice President of Operations at PharmaCircle LLC. He earned his BS in Chemistry and Mathematics from the University of Wisconsin Stevens Point. Prior to joining PharmaCircle in 2003, he held various R&D Scientist positions within Searle/Pharmacia’s Pharmaceutical Sciences Department in Analytical Development and Drug Delivery.
Total Page Views: 3131