Issue:May 2023
ION EXCHANGE EXCIPIENTS - Tackling Patient Compliance With Oral Drug Formulations Using Ion Exchange Resins
INTRODUCTION
Oral administration is the most common way people take their medications. In principle, oral medications are convenient because most are easy to swallow, and patients can self-administer without the help of healthcare staff or trips to the doctors. They come in many forms; solid tablets and capsules, powders, granules, syrups, suspensions, and, more recently, chewable tablets and gummies. All delivery forms offer painless administration with a precise quantity of the active pharmaceutical ingredient (API) that can be easily stored at home.
While the majority of individuals use oral medicines without incident, they are known to have a very bitter taste that can inhibit patient compliance. Tablets and capsules are designed to be swallowed whole and, therefore, are not in contact with the taste receptors for long enough to cause a reaction to the bitter taste of many drugs. But liquids, soluble, and chewable formulations are often in the mouth much longer than solid formulations, so the API’s bitter taste can cause problems with patient compliance.
In certain patient groups, namely geriatric and pediatric, problems can also arise with solid oral formulations as they can often have difficulty swallowing the tablets or capsules.1 60% of individuals in one study reported they found it difficult to swallow tablets or capsules, and 69% admitted to not taking a medication because it was difficult to swallow.2 To combat this, many medications for these individuals come in easy-to-administer forms such as liquids or soluble tablets. However, this then causes issues with taste, with an excess of 90% of pediatricians reporting that a drug’s taste and palatability are the most significant barriers to patients completing the treatment.3 For geriatric and pediatric patients, the bitter taste and the ability to easily take medications are significant factors in patient compliance.
THE USE OF ION EXCHANGE RESINS
Ion exchange resins (IERs) have been used for many years to aid in pharmaceutical formulations, and one of the main attributes is flexibility for the development of controlled-release formulations, such as suspensions, tablets, capsules, and orally disintegrating tablets (ODTs). These polymers are capable of exchanging ions within a solution that is passed through them. IERs enable taste-masking of bitter drugs, stability of unstable materials, and solubility enhancement of poorly soluble drugs.
In pharmaceutical applications, this functionality can be used to create more powerful, functional excipients with a direct impact on the availability of a drug and patient compliance. Drugs can be loaded onto the resins by an exchanging reaction, forming a drug-resin complex (resinate). The drug is released from the resinate by exchanging with ions in the gastrointestinal fluid, rather than in the mouth, followed by drug diffusion. Because the API is not released in the mouth, no bitter taste is registered. As they are high molecular weight water-insoluble polymers, the resins are not absorbed by the body and are therefore inert.
APIs require different drug delivery systems, from edibles such as gummies to taste-masked, controlled-release suspensions. IERs enable effective drug formulation in a broad range of uses from tablet disintegration and solubility enhancement to controlled API release and improved taste.
The versatility of IERs enables them to be exploited more widely as drug delivery vehicles for oral formulations like tablets, capsules, and syrups. In addition, by selecting specific ion exchange chemistry and platforms, it is possible to develop better patient experiences and outcomes.
MASKING THE BITTER TASTE
Humans have evolved to use taste as one of the first tests to determine if a substance is edible as opposed to potentially toxic. Therefore, it’s no surprise patients dislike and avoid taking medication when it tastes unpleasant.
There are many ways to mask the bitter taste – adding flavors or sweeteners, encapsulating or coating the API, altering the pH of the API, or using IERs. The most appropriate method is dependent upon the specific API, degree of bitterness, intended final form, and the manufacturing process. The inherent bitterness of some APIs cannot be masked by adding flavors or sweeteners alone. In addition, adding sweeteners may be undesirable for patients with diabetes, fructose intolerance, or aspartame intolerance (ie, phenylketonurics). Poorly adsorbed flavor additives and non-digestible sweeteners can also produce laxative effects.
Limiting dissolution of the API in a patient’s mouth is an alternative that can be achieved via API complexation using IERs. This method binds the API so the release in salivary pH is insufficient to produce a bitter taste, thus improving compliance. Additionally, buccal dissolution allows the selection of taste-masking formulations, helping manufacturers to optimize their taste-masking methods in vitro and show differences between formulations.
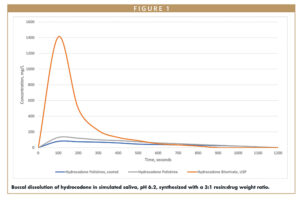
A study was conducted to demonstrate the use of IERs to mask the taste of hydrocodone, a cough and cold drug. DuPont™ AmberLite™ was used to test drug loading. These resins range from weak to strong acids and have different ionic forms – hydrogen, potassium, and sodium. Buccal dissolution was measured after loading APIs onto polistirex (AmberLite IRP69) and the effect of coating on buccal release was assessed using 10% ETHOCEL™.
The taste of hydrocodone was successfully masked by synthesizing the API with polistirex resin or coating the resinate with 10% ETHOCEL. Both formulations released significantly less API when exposed to simulated saliva than the control (Figure 1). The coated formulation achieved greater taste-masking with an efficiency of 94.3% compared to 91.7% of the polistirex alone, thus demonstrating the complementary nature of ion exchange and cellulosic coating technologies.
AIDING TABLET DISINTEGRATION & SOLUBILITY
ODTs have gained much attention as a preferred alternative to conventional oral dosage forms such as tablets and capsules. They are designed to disintegrate rapidly in the mouth upon contact with saliva and allow oral drug delivery with little chewing or the need for water. ODTs release the API in the mouth for absorption through local oromucosal tissues and through pregastric (eg, oral cavity, pharynx, and esophagus), gastric (ie, stomach), and postgastric (eg, small and large intestines) segments of the gastrointestinal tract. The administration of ODTs circumvents problems such as difficulty in swallowing traditional solid oral dosage forms. Therefore, they offer increased convenience and ease of use with the potential to improve patient compliance – especially in certain patient populations.
IERs can be used to enhance the solubility and dissolution of poorly soluble drugs. In the case of poorly soluble ionizable drugs, the release of the API from a resinate can be faster than the rate of dissolution of the solid form of the drug. IERs provide a unique solution to control API release in tablets and liquid suspensions and can be combined with other excipients to provide targeted release profiles.
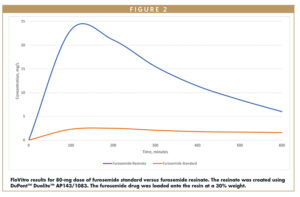
In addition, a drug’s efficiency depends on the speed at which the tablet disintegrates in the stomach. IERs that swell easily in an aqueous medium can rapidly disintegrate the tablet by creating internal pressure. Certain IERs show a minimal adherence rate, and therefore are more effective for such formulations. When looking at the release characteristics of the furosemide standard alone, it has very poor solubility, so no bioavailability of this material is seen. But when loaded onto a resinate, there is a higher payload of amorphous drug from the formulation.
HELPING PATIENTS OF THE FUTURE
With oral formulations making up the majority of drugs on the pharmaceutical market, more effort is needed to enable certain patient groups to take advantage of the benefits they provide. To do this, alternatives to solid tablets and capsules must be a focus for drug manufacturers whether that be liquids and syrups or ODTs and chewable gummies. While these circumvent issues with swallowing, they pose more problems due to the bitter taste that they can carry.
To overcome these problems, IERs should always be considered as an integral part of a formulator’s armament in the dosage form design. Companies can use these resins to tailor products to the needs of specific segments of the population, in particular, the pediatric and fast-growing older populations of patients or create more convenient dosage forms. IERs solve various pharmaceutical formulation issues, including decreasing the bitter taste of many pharmaceutical drugs, improving patient outcomes by supporting compliance to treatment regimens, and providing new revenue streams for pharmaceutical companies.
The use of IERs can be a win for patients and the pharmaceutical industry. They can help improve the manufacture of drugs to deliver solutions that result in better outcomes for patients and life science companies alike.
AUTHOR’S NOTE
This information is based on information that DuPont believes to be reliable. It is subject to change as additional knowledge and experience are gained. It is not intended as a substitute for any testing you may conduct to determine for yourself the suitability of our products for your particular purpose. Because conditions for use are outside the DuPont’s control, DuPont make no warranties, express or implied, and assumes no liability in connection with the use of this information. This information is not intended as a license to operate under or a recommendation to infringe any trademark, patent, or technical information of DuPont or other persons covering any material or its use.
REFERENCES
- Sharma V. Ion Exchange Resins and Their Applications. Journal of Drug Delivery and Therapeutics. 2014;4(4). doi:10.22270/jddt.v4i4.925.
- Strachan I, Greener M. Medication-related swallowing difficulties may be more common than we realise. Pharmacy in Practice. 2005;15 (10) 411-415.
- Milne CP, Bruss JB. The economics of pediatric formulation development for off-patent drugs. Clin Ther. 2008;30(11):2133-2145.

Amie Gehris earned her degree in Biology from Edinboro State University, PA, US. Since then, her career has spanned The Rohm and Haas Company, The Dow Chemical Company, and now DuPont. Since 1999, she has supported pharmaceuticals and bioprocessing in R&D roles, quality control, technical support, and application development. Currently, she is the Key Account Manager for the Life Sciences and Specialty business in DuPont in North America. In addition, she’s the Technical Service Manager for Ion Exchange Excipients and is the Subject Matter Expert (SME) for AmberLite™ and Duolite™ ion exchange resins globally.
Total Page Views: 6464