Issue:October 2018
WEARABLE INJECTORS - BD Wearable Drug Delivery Devices: An Attractive Proposal
THE CHANGING DRUG DELIVERY PARADIGM
Recent years have seen groundbreaking advances in pharmaceutical development, with increasingly innovative medicines being brought to market every day. However, the cost and complexity of these novel drugs has intensified the pressure to shift medication administration from traditional settings to more cost-effective alternatives. One such alternative is the patient’s own home, where life-altering molecules are now regularly self-administered subcutaneously to treat chronic diseases, such as rheumatoid arthritis, multiple sclerosis, and dyslipidemia among others.
Pharmaceutical companies have worked to develop highly concentrated monoclonal antibodies to improve treatment options for these chronic diseases.1 At the same time, they are looking to ease the burden on patients by reducing injection frequency and enabling home-based delivery. Although this new paradigm holds tremendous potential, it also brings new challenges in drug delivery, which require innovative solutions to effectively address them.
LIMITATIONS OF CONVENTIONAL DELIVERY SYSTEMS
Historically, delivering the small molecule drugs developed to treat conditions such as infection, hypertension, and hyperlipidemia was of little concern, as most of these medicines could be administered orally. Moreover, when the oral route was not an option, most traditional therapies could be easily solubilized and delivered via intravenous (IV), intramuscular (IM), and/or subcutaneous (SC) injection in a relatively small volume of fluid. Recent developments in biotechnology have produced a plethora of protein-based molecules (eg, mAbs) that must be injected to achieve their therapeutic effects. To accommodate the volume limitations of current IM and SC delivery methods, manufacturers must concentrate these mAbs thereby creating an additional challenge of high- viscosity formulations.2
This trend poses a fundamental problem with two possible solutions, addressed individually or together: 1) increase the injection volume, or 2) increase the injection duration. While these options may be feasible for IV administration, they pose significant impediments to SC delivery, especially when administered by a caregiver or a patient. Physiologically, the SC tissue has a limited physical and absorptive capacity for large volumes (eg, >10mL), and associated injection pressure may lead to drug leakage and injection pain.3-6 Thus, the clear majority of commercially available delivery devices (ie, prefilled syringes and autoinjectors) are designed to administer small drug volumes (1-2 mL) in under 15 seconds. Practically speaking, humans have a finite ability to self-inject over long periods of time with traditional delivery devices, as fatigue and the ability to hold the injection device in place waver.
WEARABLE INJECTORS PRESENT A SOLUTION
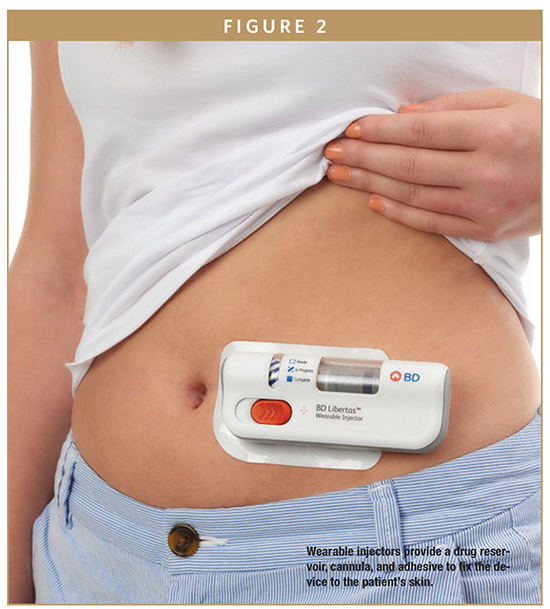
Wearable injectors (WIs) are delivery systems that adhere to the body to administer larger volumes (more than 2 mL) of drug subcutaneously over an extended period. For more than a decade, numerous pharmaceutical and medical devices companies have led development efforts to bring WIs to market, including BD LibertasTM large-volume wearable injector (Figure 1). While there is variability amongst products, all WIs provide a reservoir for the medication, a cannula for delivery to the tissue, adhesive to fix the device to the patient’s skin, and a drive system to deliver the appropriate drug volume.

WIs effectively address the volume and viscosity challenges of prefilled syringes and autoinjectors, allowing highly concentrated drugs to be diluted into larger volumes and administered over longer periods of time (minutes rather than seconds) without saturating the SC space. Although the potential benefits of these delivery systems are numerous, perhaps the most notable is the ability to self-administer high-volume, high-viscosity drugs in a nonclinical setting (Figure 2).
THE VALUE OF EXPERIENCE
Like all drug delivery devices, a successful WI must be designed to meet the needs of a variety of healthcare stakeholders. Most importantly, the WI must meet patients’ needs for simplicity in the non-clinical setting. However, WIs must also meet the pharmaceutical manufacturer’s needs for a solution that offers proven, well-integrated components that fit into existing fill/finish processes. This is a significant requirement that demands partners with experience in producing drug delivery devices.
As a leader in delivering high-quality medical devices for more than 100 years, BD can leverage its broad experience to effectively meet these requirements and introduce new drug delivery systems. BD’s extensive expertise in medical device development, primary containers, and needles allows for the seamless addition of a WI to any pharmaceutical partner’s portfolio.
BD LIBERTASTM, THE NEXT GENERATION OF WEARABLE INJECTORS
BD Libertas is a pre-assembled, fully integrated, mechanical WI designed to deliver 2-10-mL doses of high-viscosity biologics of up to 50 cP. BD Libertas’ unique design and interface were informed by extensive preclinical and clinical research, resulting in a WI with minimal steps and little complexity.
Simplicity in Design
Unlike other WIs, BD Libertas does not require user assembly or filling, significantly reducing the potential for human error and contamination. Devices that require user assembly and filling introduce the potential for dropping (and breaking) the primary container, incorrect assembly, touching aseptic areas, and increasing patient and caregiver confusion. Conversely, BD Libertas comes completely pre-assembled and ready-to-use out of the package, eliminating the greatest source of contamination: human interaction.
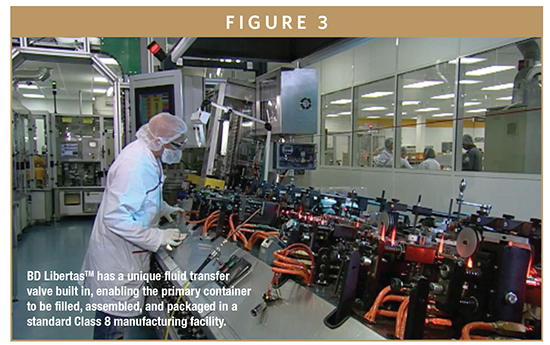
This convenient presentation is enabled by a unique fluid transfer valve built into the injector. The valve enables the primary container to be filled, assembled, and packaged in a standard Class 8 manufacturing facility (Figure 3).
PIONEERING INJECTION RESEARCH
BD has conducted rigorous preclinical and clinical research to ensure effective SC delivery of large-volume injections. The Translational Sciences Center of Excellence at BD Technologies has partnered with BD Pharmaceutical Systems to provide in vivo testing of BD Libertas. This collaboration provides valuable insights to directly impact device design, and offers early information on performance in a living system that is not easily replicated on the bench.
Approximately 40 preclinical studies were conducted to characterize the tissue response to large-volume SC deposition, investigate effects that could influence patient perception of the device, and optimize design and system components. These studies evaluated the device performance across a broad range of injection conditions that pharmaceutical manufacturers may need to deliver their molecules (eg, varying viscosities, flow rates, injection times, or body locations). One extraordinarily valuable aspect of in vivo testing is the ability to develop a model that is a good predictor of human outcomes. With rigorous preclinical testing, BD can quickly gain the information it needs to understand delivery dynamics and device footprint, and optimize device performance before moving on to human testing.
BD has used this extensive preclinical research to inform four in-human clinical studies. Two of these studies were specific to BD Libertas design component optimization, while the remaining were large-volume injection studies that employed a surrogate system to mimic BD Libertas delivery. Through these clinical studies, BD gained a comprehensive understanding of the large-volume SC injection experience across a variety of injection conditions and valuable insight into patient acceptance and preference. It’s important to provide the best possible experience for end users. Optimizing performance early saves time in the development process and gives a much better understanding of users’ needs, sooner.
INTEGRATING TRUSTED COMPONENTS
Paired with these novel innovations and capabilities, BD leverages the technologies it already delivers to pharmaceutical manufacturers by the millions every day. The BD Libertas incorporates BD NeopakTM primary container technology and employs the same cannula technology found in BD’s world-class needles. BD Libertas was purpose-built to provide a complete solution, anticipating both patient and manufacturer needs.
BENEFITS OF MECHANICAL SYSTEMS
A mechanical drive system, like that found in BD Libertas, provides a robust, industry-tested method of delivering medication. Purely mechanical systems provide reliable and known mechanisms for administration, which may help to reduce risk and increase reliability. In contrast, electromechanical devices typically require pumps, which may introduce technical complexities and unknown sources of error.
Moreover, purely mechanical devices may deliver more comfortable injections compared to electromechanical devices, as they are responsive to tissue back-pressure. As fluid diffuses into the subcutaneous space, pressure in the tissue slowly builds, which may induce pain at the injection site. When this occurs during mechanical delivery, the device responds by naturally slowing the medication delivery toward the end of the injection, reducing the potential for pain. Conversely, electromechanical devices are designed to deliver medication at a constant delivery rate regardless of tissue back-pressure.
A final advantage of purely mechanical devices is simply the absence of electronics from the core device. This is particularly beneficial when it comes to device disposal.
CUSTOMIZATION OPTIONS
BD offers the ability to adapt several aspects of the BD Libertas device, including the look and feel and injection volume, while keeping the core footprint standardized. The WI will be available in two volume formats, 2-5 mL and 5-10 mL, both housed within a similar device design.
BD Libertas’ design features customizable outer-facing components, enabling further flexibility without impacting the functionality of the device. For example, grip and button colors can be changed to reflect branding. The device’s outer cover can also be modified with components that contain enhanced functionality. In this way, any BD Libertas device can be easily modified or upgraded as needed, without any changes to the core device module.
FLEXIBILITY TO BECOME “SMART”
The BD Libertas design is futureproofed to meet evolving industry trends. More developers are looking to enhance the injection experience by incorporating “smart” features and connecting with the digital health ecosystem (Figure 4). Although a limited number of commercially available drug delivery devices currently have smart features, connected devices are poised to become the norm throughout the next 5 to 10 years.7

BD believes that smart devices should encompass both local and global connectivity: local, in that a smart device should help facilitate better interactions with individual users, and global, in that the device should enable communication with others about its state and usage. BD has taken this approach in the development of BD Libertas, while also recognizing that not every situation requires the same degree of connectivity.
BD Libertas was designed from the outset with the capacity for smart features, simply by adding a smart module to the core device. In this way, one platform can accommodate both local and global connectivity for the same molecule or across molecules within one customer. BD Libertas truly offers a platform solution for pharmaceutical companies.
SUMMARY
Wearable injectors present a robust solution to the challenge of delivering SC injections of increasing dosing volumes and viscosities in non-clinical settings. Introducing robust, innovative technologies will allow more patients to enjoy the convenience of injecting at home. In addition, the ability to accommodate new formulations with higher volume and/or viscosity will enable less-frequent injections, improve the patient experience, and potentially increase adherence to therapies.
However, bringing new injection technologies to market introduces complexities that pharmaceutical companies must consider as they select the right wearable injector platform for their portfolio. Experience in providing prefillable injection technologies, delivering well-integrated primary container and device systems, and working with partners who understand the intricacies of delivering drugs into the subcutaneous space all help to increase peace-of-mind for pharmaceutical companies in bringing combination products to market.
BD Libertas represents the newest addition to BD’s platform of integrated device components to support the development of combination products to enable a variety of options for delivering self-administered biologics.
BD LibertasTM is a product in development; some statements made are subject to a variety of risks and uncertainties.
REFERENCES
- Li L, Kumar S, Buck PM, et al. Concentration dependent viscosity of monoclonal antibody solutions: explaining experimental behavior in terms of molecular properties. Pharm Res. 2014;31(11):3161-3178.
- Galush WJ, Le LN, Moore JMR. Viscosity behavior of high-concentration protein mixtures. J Pharm Sci. 2012;101(3):1012-1020.
- Buxton ILO. Pharmacokinetics: The Dynamics of Drug Absorption, Distribution, Metabolism, and Elimination. In: Brunton LL, Knollmann BC, Hilal-Dandan R, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13e. New York, NY: McGraw-Hill Education; 2017.
- Doughty DV, Clawson CZ, Lambert W, Subramony JA. Understanding subcutaneous tissue pressure for engineering injection devices for large-volume protein delivery. J Pharm Sci. 2016;105(7):2105-2113.
- Heise T, Nosek L, Dellweg S, et al. Impact of injection speed and volume on perceived pain during subcutaneous injections into the abdomen and thigh: a single-centre, randomized controlled trial. Diabetes Obes Metab. 2014;16(10):971–976.
- Jørgensen JT, Rømsing J, Rasmussen M, Møller-Sonnergaard J, Vang L, Musæus L. Pain assessment of subcutaneous injections. Ann Pharmacother. 1996;30(7-8):729-732.
- Krishnamurthy R, Sastry A, Balakrishnan B. How the Internet of Things Is Transforming Medical Devices. Cognizant; 2016.
To view this issue and all back issues online, please visit www.drug-dev.com.

Beth DiLauri is Director, Strategic Marketing at BD Medical – Pharmaceutical Systems, responsible for developing portfolio strategies and leading commercialization for self-injection devices with the Pharmaceutical Systems business. She has dedicated her 18-year career at BD to developing and executing portfolio strategies based on deep market and customer insights, across multiple segments of the healthcare industry, including pharma/biotech, medical devices, diagnostics, and healthcare IT. Prior to BD, she was responsible for business development at Transcend Therapeutics, a venture-backed development-stage pharmaceutical company, from inception through its Initial Public Offering on the NASDAQ in 1997. She earned her MBA from the Tuck School of Business at Dartmouth College (Hanover, NH, US), and Bachelors’ degree in Psychology from Boston College (MA, US).
Total Page Views: 14128










