Issue:November/December 2020
VACCINE DEVELOPMENT - COVID-19 Vaccine Focusing on T Cells to Protect the Most Vulnerable
INTRODUCTION
As the COVID-19 pandemic persists globally, hundreds of parallel efforts are in place to develop safe and effective vaccines against SARS-CoV-2, the virus responsible for COVID-19. While most vaccines in development are targeted to protect healthy people, few are focusing on those who are most at risk. Two demographics demonstrating especially severe complications and high mortality rates are seniors and those with co-morbidities like heart failure, obesity, or type 2 diabetes.1 Additionally, most seniors, as well as some people with co-morbidities who are immunocompromised, are less likely to gain full protection from standard vaccines. For seniors, this is because the immune system declines with age.2 In particular, the immune system loses some of its capacity to maintain immunity to a given virus even after being vaccinated against it or becoming immune to it — as in the case of shingles, the painful condition resulting from reactivated chicken pox virus, in which seniors need a vaccine to avoid developing the disease even after having had chicken pox as a child. Similarly, in the case of COVID-19, even after receiving a traditional vaccine, seniors may risk developing virus symptoms if they encounter SARS-CoV-2 months or years later.
FOCUSING COVID EFFORTS ON SENIORS WILL GO A LONG WAY
With these challenges in mind, Heat Biologics is collaborating with the University of Miami Miller School of Medicine to develop a COVID-19 vaccine designed specifically for seniors and those with co-morbidities, the groups who are both especially vulnerable to COVID-19 and who are less likely to respond to traditional vaccines. Our vaccine is designed to add an extra layer of protection in the form of long-term T cell-mediated immune memory. If the vaccine is ultimately approved, it can be given along with other available vaccines as a second line of defense, or it may be given some time after initial vaccination as a booster shot.
We’ve built a technology platform for use against cancer and infectious disease based on an immunostimulatory protein called gp96. As the COVID-19 pandemic began escalating in early March, we quickly co-opted our existing gp96 platform to target the SARS-CoV-2 virus in addition to continuing ongoing clinical-stage programs in cancer.
GP96 HARNESSES T CELL-MEDIATED IMMUNE RESPONSES
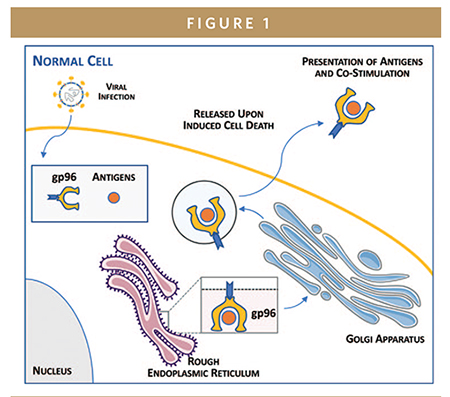
In the human immune system, gp96 is a chaperone protein — a protein that helps newly made proteins fold into active configurations. It also serves as a potent immune stimulator, or adjuvant, and immune memory inducer. gp96 is normally tethered to the inside of the cells it inhabits and is only released from cells in the event of cell death, or necrosis. Upon necrosis, gp96 molecules display/deliver any proteins or antigens they are carrying to the immune system’s dendritic cells. Those dendritic cells stimulate a powerful, specific, and lasting B cell- and T cell-mediated immune response to those antigens.
Necrosis is brought on by adverse conditions, like infection, in the cell, so gp96 acts to “alert” the immune system to agents like tumor markers or viral proteins that may have caused a given cell’s death. In this way, gp96 functions as a potent immune warning system that induces immunity against necrosis-causing agents like cancer or pathogens. Importantly, by activating and expanding B and T cell populations, gp96 can upregulate both humoral (antibody) and cell-based immunity, respectively: B cells produce antibodies against a pathogen, while T cells differentiate into both CD8+ T cells that can kill pathogens and CD4+ T cells that “remember” pathogens upon later exposure.
GP96 IN CANCER
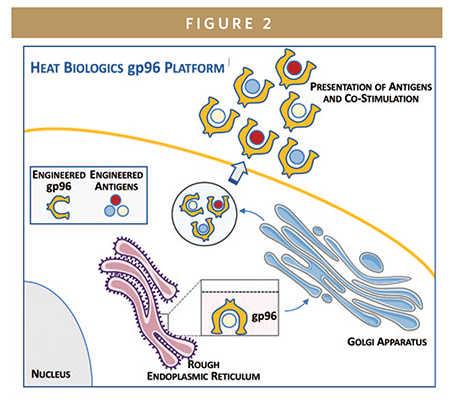
Our proprietary gp96 vaccine platform comprises whole cells, injected under the skin, that continually secrete a modified version of gp96 that can leave the cell at any time. These cells also continually express antigens of interest, related to a cancer type or infectious disease, which gp96 molecules bind and carry out of the cell. As with gp96’s native behavior, gp96 secreted by any of Heat Biologics’ vaccines display those bound antigens to the dendritic cells that traffic at high density under the skin, thereby eliciting a powerful immune response.
Heat Biologics’ gp96 platform underlies the company’s lead cancer immunotherapy programs: its Phase 2 asset (HS-110) is currently being evaluated in non-small cell lung cancer, and its Phase 1 asset, HS-130, is being evaluated in solid tumors. Altogether, the platform has been tested in over 300 patients in clinical trials, with broad T cell activation shown to date.
CO-OPTING PLATFORM TO FIGHT COVID-19 THROUGH T CELLS
The gp96 platform can be co-opted to target a wide variety of cancers or infectious diseases by transfecting a cell line already expressing Heat’s modified gp96 with a carefully chosen set of antigens from each target disease of interest. In the case of Heat Biologics’ COVID-19 vaccine program, selected viral antigens from SARS-CoV-2 were transfected into a proprietary human cell line.
This is not the first time that Heat Biologics’ technology has been harnessed in infectious disease rather than cancer: it has also been co-opted in National Institutes of Health (NIH)- and Department of Defense (DOD)-funded mouse and non-human primate trials in simian immunodeficiency virus/HIV, Zika virus, and malaria. All studies demonstrated that the gp96-based technology stimulated both B and T cell-mediated immune responses in different target tissues and organs, including the gut, reproductive tract, and liver.
When Heat Biologics began a COVID-19 vaccine collaboration in March of this year, the company joined forces with a team of scientists led by Natasa Strbo, MD, DSc, research assistant professor of microbiology and immunology at the University of Miami Miller School of Medicine. Dr. Strbo has co-developed the gp96 platform and led the aforementioned animal studies in infectious disease.
At the outset, Heat Biologics’ COVID-19 vaccine candidate was developed with findings from previous immunological studies in mind in order to help populations like seniors and the immunocompromised to achieve long-lasting immunity they would otherwise be unlikely to attain. While most COVID-19 vaccines in development — and, in fact, most vaccines in immunity primarily through activating B cells that produce antibodies against the virus, such immunity tends to be either relatively short-lived or insufficient for full immunity.
In the case of COVID-19, antibody levels in the blood have been shown to wane on the scale of months after infection.3 Such candidates fail to account for the importance of longer-term adaptive immunity provided by activated T cell populations, which is also the component of immunity that is known to decline with age in the absence of specific boosts from vaccines. T cell responses are critical for directly killing virus-infected cells, producing highly specific antibodies and for long-term immune memory on the scale of years.4 An ideal vaccine, particularly for seniors and the immunocompromised, would provide long-term immunity by stimulating a coordinated response between both the innate and adaptive arms of the immune system, in which “helper” CD4+ T cells aid B cells in producing highly specific antibodies while activating “killer” CD8+ T cells that kill virus-infected cells.5 Memory T cells are likely crucial for long-term immunity as well, as suggested by their correlations with patients who recovered from infections with SARS-CoV-1, a related coronavirus outbreak, in 2003.6
Heat Biologics’ scientists relied on their own experience in developing a product that stimulates such a coordinated response: the company’s gp96-based cancer immunotherapy candidate HS-130 activates both CD4+ and CD8+ T cells as well as antibodies that neutralized infection. Similarly, Heat’s COVID-19 vaccine candidate is designed to protect against COVID-19 infection if people are exposed to the virus months or even potentially years after vaccination.
Notably, as several developers advance COVID-19 vaccine candidates in parallel, multiple vaccines will likely become available to the public in a staggered fashion, meaning that people may face uncertainty about whether to try the first vaccine available to them or to wait for subsequent options. Heat Biologics’ vaccine is designed be administered either in place of or in addition to other vaccines, as a primary or as a booster, thereby complementing or adding to other vaccines’ strengths as needed; this particular candidate will not add complexity to a person’s choices for themselves or their families.
PRECLINICAL DATA SHOWS T CELL EXPANSION
Since March, Heat Biologics and UM collaborators have already designed and optimized the vaccine and conducted preliminary in vitro and in vivo studies. Recent results from a study in mouse models are highly encouraging, demonstrating robust T cell-mediated immune responses directed against SARS-CoV-2’s S glycoprotein, or spike protein.
After a single injection, the vaccine induced the expansion of both “killer” and “memory” CD8+ T cells, as well as “helper” CD4+ T cells, which aid in producing antibodies. Importantly, the memory CD8+ T cells in particular were found to migrate to the lungs and airways – the tissue specific sites of interest for SARS-CoV-2 infection. Such mucosal immunity is critical in mounting an immune response to respiratory viruses like SARS-CoV-2. Taken together, these results meet several requirements for further study, and potentially offer long-lasting T cell-mediated immunity for people who need it most.
MOVING FORWARD
With preclinical data in hand, Heat Biologics has made preparations for manufacturing the vaccine at sufficient scale to conduct clinical trials — a non-trivial matter when it comes to cell-based products, which require especially delicate scale-up and preservation. The company is partnering with Waisman Biomanufacturing, part of the Waisman Center at the University of Wisconsin-Madison, for Phase 1 and 2 clinical trials. Phase 1 trials could begin in early 2021, and UW–Madison may also serve as a trial site. Waisman previously produced therapies for Heat Biologics’ clinical trials, so preparations are already in place to quickly begin producing a COVID-19 vaccine.
The company aims to test the vaccine in humans, beginning with standard Phase 1 trials in healthy volunteers. Clinical trial administrators will collect as much data through remote or virtual methods as possible using telemedicine appointments and electronic surveys, in alignment with current FDA guidance. Once safety is established in the healthy volunteers, the company plans to narrow its focus to seniors and high-risk people with co-morbidities by enrolling subjects through veteran affairs hospitals, and large hospital systems in the US to ensure that the vaccine can be evaluated in its target demographics, not just baseline healthy subjects.
CONCLUSION
As COVID-19 continues to spread, it disproportionately harms seniors and those with co-morbidities, who are unlikely to gain protective immunity from many of the vaccines currently in development. We are advancing a COVID-19 vaccine designed to overcome the immune deficits that make those populations vulnerable to the disease. The vaccine is designed to stimulate a coordinated adaptive immune response by focusing on a T cell expansion in addition to immediate antibody production, with the ultimate objective of generating long-term immunity for people who need it most. u
REFERENCES
- COVID-19 Hospitalization and Death by Age. (n.d.). Retrieved September 01, 2020, from https://www.cdc.gov/ coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html.
- Montecino-Rodriguez, E., Berent-Maoz, B., & Dorshkind, K. (2013). Causes, consequences, and reversal of immune system aging. The Journal of clinical investigation, 123(3), 958–965. https://doi.org/10.1172/JCI64096Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. 2020.
- Ibarrondo, F. J., Fulcher, J. A., Goodman-Meza, D., Elliott, J., Hofmann, C., Hausner, M. A., Ferbas, K. G., Tobin, N. H., Aldrovandi, G. M., & Yang, O. O. (2020). Rapid Decay of Anti-SARS-CoV-2 Antibodies in Persons with Mild Covid-19. The New England journal of medicine, NEJMc2025179. Advance online publication. https://doi.org/10.1056/NEJMc2025179.
- Crotty S. (2015). A brief history of T cell help to B cells. Nature reviews. Immunology, 15(3), 185–189. https://doi.org/ 10.1038/nri3803.
- Thevarajan, I., Nguyen, T., Koutsakos, M., Druce, J., Caly, L., van de Sandt, C. E., Jia, X., Nicholson, S., Catton, M., Cowie, B., Tong, S., Lewin, S. R., & Kedzierska, K. (2020). Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nature medicine, 26(4), 453–455. https://doi.org/10.1038/s41591-020-0819-2.
- Le Bert, N., Tan, A.T., Kunasegaran, K. et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 584, 457–462 (2020). https://doi.org/10.1038/ s41586-020-2550-z .

Jeffrey Wolf is Founder, Chairman, and CEO of Heat Biologics. He also founded Seed-One Ventures, a firm focused on the systematic formation and management of new biomedical companies based upon breakthrough research. Throughout his career, Mr. Wolf has specialized in building new life-science companies from the ground up and has played an active role in supporting the growth of his companies. Mr. Wolf’s start-ups include Avigen (Co-founder and Director), a NASDAQ-listed gene therapy company; TyRx Pharma (Co-founder and Chairman), which was focused on the development of novel bio-compatible polymers and recently sold to Medtronic and EluSys Therapeutics (Founder and CEO), focused on the development of novel antibodies against infectious diseases. Mr. Wolf earned his MBA from Stanford Business School, his JD from New York University School of Law, and his BA from the University of Chicago, where he graduated with honors in Economics.
Total Page Views: 7780










