Issue:March 2024
VACCINE DEVELOPMENT - A Healthier Global Population Through a Universal Influenza & Multi-Virus Vaccine
INTRODUCTION
The global vaccine market was put into a tailspin and tested to its limits with the advent of the Covid-19 pandemic. While the world spent almost 3 years grappling with a severe health calamity (and, to an extent, continues to do so), Covid-19 vaccines were quickly developed to combat the high rates of sickness and death. But, we can’t forget the infectious diseases that we were facing before the pandemic and continue to contend with today: influenza, RSV, and tuberculosis, among others. All continue to be public health crises. Seasonal influenza alone can cause anywhere between 290,000 to 650,000 global deaths each year.1 With this reality as backdrop, the US needs to address how the vaccine technology of the future is going to overcome the current challenges of efficacy and durability to ensure we re-establish faith in vaccines and make certain that all members of society have access to protection. More specifically, we need to have an eye toward universal influenza or multi-virus vaccines that have the ability to provide broad coverage as viral strains continue to evolve.
RECENT GOVERNMENT RESPONSES TO INFECTIOUS DISEASES
In August 2023, the US Government announced a new agency with focus on global health and pandemic prevention: The Bureau of Global Health, Security and Diplomacy.2 The purpose of this agency is to organize diplomacy, foreign assistance, and national policies to be better prepared for infectious diseases and future pandemics. While the mandate for the agency is quite broad, it’s a step forward by the US Government in the aftermath of Covid-19, especially with recent global outbreaks of diseases such as mpox.
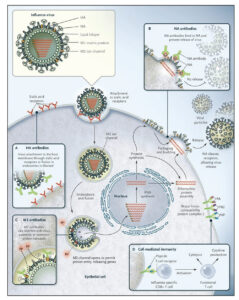
Click image to enlarge. Vaccines composed of peptides targeting multiple epitopes produce an immune response that interferes with several stages of the influenza virus life cycle simultaneously such that broad protection can be achieved. The key conserved epitopes produce neutralizing antibodies specifically targeting the ability of influenza virus to attach to host membranes (HA antibodies – Panel A), replicate within cells (M2e antibodies – Panel C), and be released from the host cell (NA antibodies – Panel B). Additionally, T cell activation induces cell-mediated immunity for direct killing of infected cells and further coordination of virus clearance (Panel D).
When Covid-19 first spread within the US, the government enacted Operation Warp Speed (another ambitious initiative), which was a partnership between the Department of Health and Human Services and the Department of Defense to accelerate the development of a Covid-19 vaccine.3 To ensure the highest rate of success under Operation Warp Speed, drug companies took steps to set-up large-scale manufacturing while running clinical trials to get quicker vaccine approval from the US FDA. As a result, we saw vaccine approvals for Pfizer, Moderna, and Johnson & Johnson. Today, more than 64% of the global population has been fully vaccinated, with the US donating more COVID-19 vaccines than any other country.4 With the efficacy data that spans the past 3 years, we have greater insight into what works and what doesn’t.
Developing and delivering a vaccine in the middle of pandemic remains challenging. The delay in immunizing the public causes social and economic damage. For viruses such as Covid-19 and influenza, time from development to delivery allows the virus to mutate. Continued morbidity and mortality from reduced efficacy and lack of durability can cause vaccine fatigue to the point where people choose not to get booster shots and additional vaccines — even if they need them.
SHINGRIX: A CASE STUDY IN SUCCESSFUL VACCINE DEVELOPMENT
Shingrix, an adjuvated recombinant protein vaccine for shingles developed and sold by GlaxoSmithKline (GSK), replaced Zostavax, a predecessor to Shingrix that was less efficacious and had shorter durability. Zostavax was discontinued in the US 3 years after the launch of Shingrix, which opened the opportunity to target more of the shingles market for GSK.
Unlike Zostavax, which delivered the entire varicella zoster virus, Shingrix delivered a key protein along with a potent liposomal adjuvant. The role of adjuvants is to serve as immune activators, heightening the body’s recognition of the vaccine target and facilitating a more robust and longer-lasting protective response.
With such technology behind it, Shingrix revealed itself as a good example of an adjuvated recombinant protein vaccine with characteristics that developers should strive to achieve across other vaccines.
THE SEARCH FOR BROAD INFLUENZA VACCINES
Scientists in the anti-viral medicine space have worked for decades to find a target within the influenza virus that is conserved and immunologically important across multiple strains to overcome the virus’ rapid mutation. The most common focus is on conserved regions of hemagglutinin to prevent binding to the cell surface. The Hemagglutinin Inhibition Assay has been the standard release criteria for the annual influenza vaccine. Another key target has been the matrix protein, a key component in viral replication of the virus. A t-cell epitope is attached to the matrix epitopes to enhance cytokine activity and improve clearance. New interest has developed in the neuraminidase (NA). The NA is important to release the influenza virus from an infected cell. A t-cell epitope is added to this target as well to enhance cytokine activity to improve clearance.
Seasonal vaccines currently offer the best protection against influenza as it is an endemic disease that follows a predictable seasonal pattern: it causes outbreaks during colder months and recedes in warmer seasons. While access to seasonal vaccines is generally good, the issue is that these vaccines tend to lag against the most current influenza strain. The yearly influenza vaccine is designed based on circulating strains from the prior season and circulating strains in the Southern Hemisphere 3-to-6 months prior to influenza season in the US. These predictions aren’t always perfect, and the virus can change and mutate quickly, rendering the vaccines less effective. The significant annual variation in efficacy builds a strong case for a universal influenza that would generate consistent efficacy from season to season.
DEVELOPING A UNIVERSAL INFLUENZA VACCINE
Unlike seasonal influenza vaccines, a universal vaccine doesn’t target influenza broadly. The vaccine is targeted to one or more key parts of the virus that are common across strains. By focusing on these more stable regions of important proteins, the vaccine teaches our immune system to recognize and fight against the virus regardless of its unique mutations. Imagine the influenza virus as a puzzle, and the universal vaccine figures out which pieces of the puzzle stay the same. Even if the rest of the puzzle changes, the core pieces stay constant, making it easier for our immune system to identify and fight the virus. The idea is that with a universal influenza vaccine, we wouldn’t need a new vaccine every year. It could provide protection against a wide range of influenza strains, including ones that haven’t appeared yet.
To move forward, companies like Longhorn are taking different approaches to attack the virus at different stage of its lifecycle: binding and entering the cell, replicating inside the cell, and as it attempts to exit the cell. It is important the vaccine generates both cellular and humoral immunity to ensure longer efficacy. A broad immunological response as provided by t-cell epitopes and adjuvants is important to combat a virus that has demonstrated the ability to continuously evolve and overcome the immune system.
The development process for a universal influenza vaccine doesn’t come without challenges. It’s important to target more than one area of the virus, so vaccines need to incorporate multiple targets while having anti-viral activity occur both with individual targets and as a complete set. That way, the vaccine can protect against multiple strains of influenza.
To get around this challenge, Longhorn developed a patented composite peptide approach that allows epitopes to be strung together into a unique peptide. The process involves finding conserved epitopes for each target, putting them in a specific order, and including a t-cell epitope to further stimulate the immune system. Longhorn teamed up with the Walter Reed Army Institute of Research (WRAIR), one of the world’s foremost developers of adjuvants, to include ALFQ, their novel liposomal adjuvant. ALFQ, a similar but improved formulation of the liposomal adjuvant in Shingrix, has repeatedly demonstrated the ability to generate a robust balanced immune response while significantly reducing reactogenicity. This combined approach also has the potential to evolve into a multi-pathogen vaccine that combines immunity against viruses such as influenza and Covid-19, or other pathogens.
If Longhorn’s universal flu vaccine is successful, it could also be used to stop the spread of animal-borne influenza strains. The avian influenza strain H5N1 has been spreading through the US poultry industry throughout the past year, which has caused food prices to rise and increased the risk that a new pandemic virus could emerge. While currently the virus does not spread from human-to-human efficiently, an intermediate host such as pigs could allow the virus to mutate and create a novel virus. Pigs are the most likely source of new pandemic influenza strains because they are naturally infected with human and avian influenza strains.
Adjuvanted recombinant protein vaccines have decades of safety data, are easily manufactured, and are well accepted by the general population, making them ideal for large populations. Demonstrating high efficacy and long-term durability is far from certain; however, the potential benefits of enhanced immunogenicity, cross-protection potential, and improved safety profiles are compelling. As research and development efforts continue, these vaccines could play a pivotal role in reshaping the way we combat influenza through a universal vaccine, and other infectious diseases through a multi-pathogen vaccine, ultimately contributing to a healthier global population.
REFERENCES
- World Health Organization: WHO & World Health Organization: WHO. (2023). Influenza (Seasonal). www.who.int. https://www.who.int/ news-room/fact-sheets/detail/influenza-(seasonal)#:~:text=Worldwide%2C_these_annual_epidemics_are,65_or_older_(1).
- Pfeiffer, S. (2023, August 3). A new U.S. agency is a response to the fact that nobody was ready for the pandemic. NPR. https://www.npr.org/sections/goatsandsoda/2023/08/03/1191872141/a-new-u-s-agency-is-a-response-to-the-fact-that-nobody-was-ready-for-the-pandemic
- Operation Warp Speed: Accelerated COVID-19 vaccine development status and efforts to address manufacturing challenges. (2021, February 11). U.S. GAO. https://www.gao.gov/ products/gao-21-319.
- U.S. COVID- 19 global response recovery framework – the white house. (n.d.). https://www.whitehouse.gov/ wp-content/uploads/2022/09/U.S.-COVID-19-GLOBAL-RESPONSE-RECOVERY-FRAMEWORK-_clean_9-14_7pm.pdf.

Jeff Fischer is the President of Longhorn and is responsible for oversight of all non-scientific aspects of the organization to include regulatory affairs. He co-founded the company and has served as its Chief Financial Officer from 2007-2017. From 1998-2005, he served as an Executive Vice President and CFO in the biotechnology industry. He is a former infantry officer in the United States Marine Corps and earned his MBA from the University of Texas at Austin.
Total Page Views: 2514










