Issue:April 2013
THERAPEUTIC FOCUS: Sufentanil NanoTab Technology: Revolutionizing Patient-Controlled Analgesia
Introduction
Despite advances during recent years, acute pain management, particularly in the hospital setting, remains a pressing challenge and a significant unmet medical need. Up to 75% of surgical patients report inadequate pain relief following surgery, and with more than 30 million inpatient surgical procedures performed annually in the US, millions of patients endure inadequately managed postsurgical pain each year.1,2 Inadequate treatment of post-surgical pain can lead to decreased mobility, which increases the risks of other medical complications, including deep vein thrombosis.3 Additionally, severe post-surgical pain is one of the two main predictors for the development of chronic post-surgical pain (CPSP) syndrome.
Intravenous patient-controlled analgesia (IV PCA) is one of the most common methods currently utilized to help control post-surgical pain. IV PCA delivers a dose of pain medicine, typically morphine or hydromorphone, directly into the patient’s vein via a programmable pump kept at the patient’s bedside. When patients feel pain, they depress a button attached to the pump and receive a dose of medication. A lockout interval is programmed into the pump to reduce the chance of the patient overdosing. Numerous studies throughout the past few decades have demonstrated that allowing patients to control their own post-operative pain medication results in improved analgesic efficacy and patient satisfaction relative to nurse-administered analgesic medication.5,6 Patient-controlled analgesia is an important aspect of post-operative pain management as evidenced by the millions of patients in the US each year utilizing IV PCA to manage their post-operative pain in the hospital setting.7 Unfortunately, IV PCA technology is increasingly associated with significant safety concerns, including medication errors due to mis-programming of the pump, analgesic gaps due to issues with IV tubing, potential for infection due to the need for venous access, and adverse drug events due to the side effect profiles of the commonly used opioids.8-11 According to published literature, IV PCA errors occur an estimated 407 times per 10,000 people treated in the US each year.7 The most common and serious types of errors involve human factors, such as misprogramming the PCA pump or administering the wrong dose, which can lead to adverse events and even death.8 In 2002, approximately 5% of operator errors reported to the US FDA resulted in patient deaths.12
Recently, the FDA has taken notice. In 2010 it evaluated a broad spectrum of infusion pumps across manufacturers and concluded that there are numerous issues with device design, manufacturing, and adverse event reporting. Approximately 56,000 adverse events relating to infusion pumps were reported to the FDA between 2005 and 2009, prompting 70 Class II infusion pump recalls (potential for temporary or reversible adverse effects) and 14 Class I infusion pump recalls (potential for serious injury or death).13 These issues with infusion pumps have resulted in the issuance of draft guidance by the FDA.14
In addition to the complexity of the infusion pump, IV PCA safety concerns extend to the side effects associated with the drugs themselves. Published data on IV PCA side effect profiles show that approximately 50% of patients experience sedation, and oxygen desaturation has been shown to occur in approximately 10% of patients.15,16 Morphine, still utilized in the majority o post-operative IV PCA cases, has active metabolites, which are eliminated by the kidney and can lead to unanticipated pharmacokinetic-pharmcodynamic effects, such as prolonged duration of action and untoward side effects in patients with decreased renal function.17-19 Morphine can also produce somnolence, delirium, oxyge desaturation, and respiratory depression.20 Hydromorphone use in IV PCA has increased recently but has also demonstrated excessive levels of somnolence. In a systematic review of adverse events for opioids used postoperatively, hydromorphone was associated with a 43% incidence of central nervous system (CNS) side effects, mainly sedation.11 Excessive sedation in the post-operative setting can lead to respiratory depression and can slow post-operative rehabilitation and discharge from the hospital. Further, being tethered to an IV pole when using IV PCA reduces patient mobility, and along with IVrelated complications, such as infections or analgesic gaps, can further delay discharge. Complications resulting in delayed discharge are costly; a 2009 study estimated that hospitals incur $1,657 in actual costs per day for inpatient surgical patients.21
To mediate these challenges, pain management practices have evolved to reduce the amount of opioid utilized to manage postoperative pain. Adjuvant pain management methods, such as regional anesthetic techniques (e.g., epidurals, spinals, nerv blocks) and non-opioid pain relievers (e.g., acetaminophen and anti-inflammatory drugs), can reduce opioid consumption postoperatively. 22 However, they can also create their own challenges, including increased complexity and additional cost of care, and the potential for additional side effects unique to each adjuvant must be taken int consideration.23-28 Furthermore, most adjuvant analgesics lack the patient-controlled aspect of post-operative pain management, which has the potential to reduce patient satisfaction.
Patients, nurses, physicians, pharmacists, and hospital systems need a solution that can deliver the benefits of PCA in terms of patient pain relief for moderate to severe pain conditions and satisfaction while reducing both drug and device safety risks. Such a system would also represent an opportunity to reduce the overall complexity of pain management, and hence reduce the cost burden on the healthcare system. AcelRx Pharmaceuticals has developed the Sufentanil NanoTab PCA System to address these specific issues.
Opportunities for Innovation & Improvement – The Triumvirate of Safety
To enable the concept of PCA to reach its true potential for improving care and satisfaction, post-operative patients need effective, rapidly titratable pain relief combined with low risk for adverse events and medication errors. In order to achieve these goals, AcelRx looked at the three fundamental building blocks of PCA: drug, route of administration, and device. Of these, the first and most important consideration is the choice of pain medication.
Sufentanil: An Underutilized Opioid
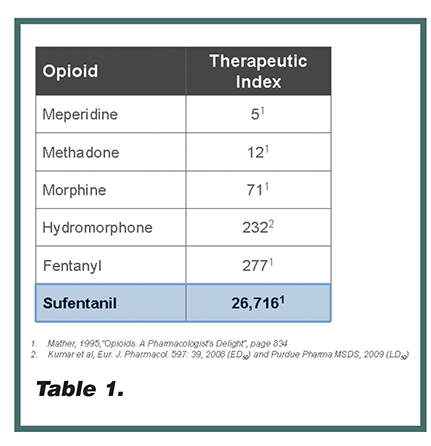
Sufentanil is an opioid that potentially offers multiple pharmacologic advantages over other opioid pain therapies commonly used to manage post-operative pain in the hospital setting. It is commonly understood that sufentanil is 5 to 10 times more potent than fentanyl and lacks active metabolites, thereby minimizing complex pharmacokinetics post-operatively.29 What is less well known is sufentanil’s high therapeutic index (Table 1). Therapeutic index is the ratio of the lethal dose to the effective dose of a drug, which is used as a measure of the relative safety of the drug for a particular treatment. Sufentanil’s therapeutic index in preclinical models is approximately 80 times higher than fentanyl and 370 times higher than morphine. From a clinical perspective, published studies demonstrate that sufentanil produces a less-frequent incidence of respiratory depression relative to its analgesic effects compared to other opioids, including morphine, alfentanil, and fentanyl.17, 30-34
These pharmacological advantages of sufentanil have the potential to reduce the risk of medication-related side effects, improve patient safety, and provide enhanced pain control. Therefore, sufentanil appears to be an optimal opioid for post-operative PCA. Yet, despite sufentanil’s established record of analgesic efficacy and safety, its use as a systemic analgesic has been limited due to its very short duration of action when delivered intravenously. Thus, AcelRx seeks to access sufentanil’s potentially improved safety and pain-relieving capability via sublingual delivery to prolong its duration of action.
NanoTab Technology: Tiny Tablet, Huge Advance
When evaluating the appropriate route of administration for PCA, it is important to consider several criteria. The route must allow patients to dose themselves even if they are unable to take oral medication, must provide a rapid and consistent onset of action, and must be amenable to repeat dosing. Ideally, the route of administration is non-invasive, patient-friendly, and does not restrict patient mobility.
Sufentanil NanoTabs are very small (3- mm diameter), bioadhesive tablets and are formulated to be non-irritating. When placed under the tongue, the Sufentanil NanoTab adheres quickly to the sublingual tissue and is largely undetectable by the patient. Because of its small size, the NanoTab generates very little salivary response – thereby minimizing reflex swallowing of saliva containing dissolved drug – and enables sufentanil to be delivered almost entirely through the sublingual transmucosal route.35 Sufentanil NanoTabs display highly consistent pharmacokinetics due to this single, highly consistent route of drug uptake, which allows for safe repeat dosing in the post-operative setting.35
Due to sufentanil’s pharmaceutical attributes, including degree of lipid solubility and ionization, uptake from the sublingual tissues is rapid, and therefore, onset of action is rapid as well – making sufentanil an ideal pain medication for post-operative patients experiencing significant pain.35,36 In addition, when delivered sublingually, sufentanil avoids the first-pass metabolism and delayed onset of oral medications and avoids the high peak plasma levels and short duration of action of IV administration, enabling patients to dose themselves as necessary to titrate to an analgesic level and maintain a consistent level of pain relief.35
The non-invasive nature of the NanoTab is patient-friendly, and removes the opportunity for complications or analgesic gaps associated with IV delivery. Further, patients need not be tethered to an IV pole to manage their pain, enabling greater patient mobility.
In order to further enhance the benefit of NanoTab technology, the Sufentanil NanoTabs have been coupled with a novel PCA delivery system to allow the patient-controlled aspect of post-operative pain management.
The Sufentanil NanoTab PCA System: Delivering Rapid, Reliable Relief
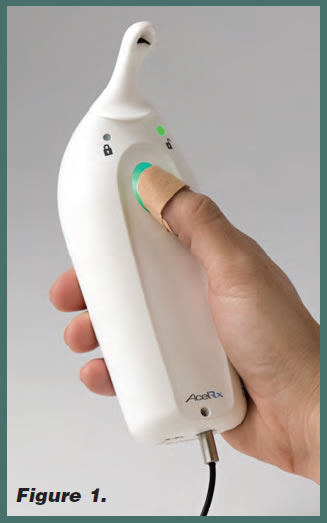
The Sufentanil NanoTab PCA System (Figure 1) is a unique, preprogrammed, handheld technology system designed to deliver all of the beneficial features of typical IV PCA while leveraging the potential benefits of sufentanil to address the need for rapid and reliable patient-controlled pain management in the hospital setting.
The Sufentanil NanoTab PCA System is preprogrammed with a fixed Sufentanil NanoTab dose of 15 mcg and a fixed 20- minute lockout interval between doses, eliminating the risk of pump programming errors, which are a potential source of patient harm with typical IV PCA technology. The patient wears a radio-frequency identification (RFID) thumb tag, which uniquely identifies that patient to the device and controls access to the medication. An RFID-controlled access card is used by healthcare professionals to set up and access administrative functions on the system. The system is designed to be simple to use for both patients and healthcare professionals, and tracks dosing history to assist in assessing appropriate use and enable enhanced drug reconciliation.
AcelRx completed human factors studies with nurses and a Phase II open-label device functionality study in patients to ensure the design and functionality of the system was easy to use and understand. In the nurse study, nurses were asked to complete a number of tasks and rate the ease of use with the system. After completing all tasks, the nurses gave the system an overall average Ease of Use rating of 4.73 and an overall average Ease of Learning rating of 4.57 using a 5-point scale (1=extremely difficult to 5=extremely easy). All thirty patients (100%) in the Phase II study reported in a questionnaire that they could “handle the system easily and the instructions were clearâ€. Results from these studies demonstrated the Sufentanil NanoTab PCA System is easy to learn and use.37-39
Clinical Results to Date
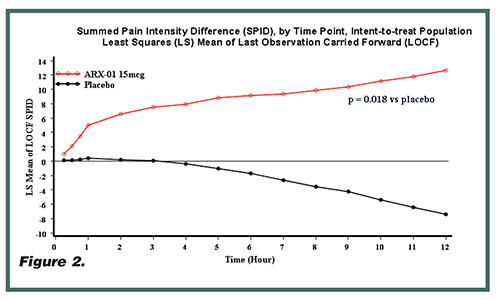
Clinical data to date demonstrate the safety, efficacy, and clinical benefit of Sufentanil NanoTabs. Three Phase II studies of Sufentanil NanoTabs have reported positive clinical results. Two of the Phase II studies were multicenter, double-blind, randomized, placebo-controlled trials, one in patients undergoing elective unilateral knee replacement surgery and the other in patients undergoing major abdominal surgery. The final Phase II study was an open-label Phase II system functionality study of the handheld component of the Sufentanil NanoTab PCA System in unilateral knee replacement surgery (Figure 2). The primary efficacy endpoint in these studies was the sum of the pain intensity differences at each evaluation time point compared to baseline over the 12- hour study duration (SPID-12). The group treated with Sufentanil NanoTabs 15 mcg showed a statistically significant reduction in pain intensity over the study period (p < 0.02 for knee surgery and p < 0.001 for abdominal surgery). These studies were conducted with no rescue opioids or adjuvant therapy, demonstrating that the majority of patients were able to effectively manage their post-surgical pain with Sufentanil NanoTabs alone.40,41
In these Phase II studies, the average time interval between doses was approximately 80 minutes.38 This compares favorably to the much shorter redosing interval, which is typical for IV PCA (20 to 40 mins).42,43 The longer redosing interval observed with Sufentanil NanoTabs not only allows patients to easily manage their pain, but allows for increased periods of mobility or sleep, which could have a positive effect on post-surgical recovery.
In all studies, Sufentanil NanoTabs were well tolerated. Patients dosed with Sufentanil NanoTabs exhibited a low incidence of sedation (3%) and oxygen desaturation (1%) at the 15 mcg dose.37,40,41 The incidence of these critical adverse events is quite low when compared to a published meta-analysis of post-operative IV PCA-induced side effects, as mentioned earlier.15,16
Future Development and Milestones
Based on these positive results, and the unmet medical need for improved patient-controlled analgesia in the post-operative hospital setting, the Sufentanil NanoTab PCA System is currently undergoing Phase III testing. The Phase III program includes two randomized, double-blind, placebocontrolled studies and one open-label active comparator study. Placebo studies include both a major abdominal and a major orthopedic surgery study, while the active comparator study is a non-inferiority study with IV PCA morphine as the comparator in both of these post-surgical populations combined.
Meeting the needs of post-operative patients in pain, and meeting those needs in a safe, effective way, is an imperative for modern healthcare. The technology behind the Sufentanil NanoTab PCA System represents a significant departure from existing therapeutic options and delivery systems, and has the potential to both improve patient care and reduce health system burdens.
References
1. Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet 2011.
2. Health Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) 2009.
3. Walder B, Schafer M, Henzi I, Tramer MR. Efficacy and safety of patientcontrolled opioid analgesia for acute postoperative pain: a quantitative systematic review. Acta Anaesthesiol Scand. 2001;45:795-804.
4. Gupta A, Gandhi K, Viscusi E. Persistent postsurgical pain after abdominal surgery: techniques in regional anesthesia and pain management. 2011;15(3):140-146.
5. Walder B, Schafer M, Henzi I, Tramer MR. Efficacy and safety of patientcontrolled opioid analgesia for acute postoperative pain: a quantitative systematic review. Acta Anaesthesiol Scand. 2001;45:795-804.
6. Ballantyne JC, Carr DB, Chalmers TC, Dear KB, Angelillo IF, Mosteller F. Postoperative patient-controlled analgesia: meta-analyses of initial randomized
7. Meissner B, Nelson W, Hicks R, et al. The rate and costs attributable to intravenous patient-controlled analgesia errors. Hosp Pharm. 2009;44:312-324.
8. Institute for Safe Medication Practices. Medication safety alert. High alert medication feature: reducing patient harm from opiates. Available at: http://www.ismp. org/Newsletters/acutecare/articles/20070222.asp. Accessed May 20, 2010.
9. Panchal SJ, Damaraju CV, Nelson WW, et al. System-related events and analgesic gaps during postoperative pain management with the fentanyl iontophoretic transdermal system and morphine intravenous patient-controlled analgesia. Anesth Analg. 2007;105:1437-1441.
10. Webster J, Osborne S, Rickard C, et al. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2010;3:CD007798.
11. Wheeler M, Oderda GM, Ashburn MA, et al. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3:159-180.
12. Hankin CS, Schein J, Clark JA, Panchal S. Adverse events involving intravenous patient-controlled analgesia. Am J Health-Syst Pharm. 2007;64:1492-1499.
13. Center for Devices and Radiological Health, US Food and Drug Administration Infusion Pump Improvement Initiative. April 2010.
14. CDRH. Total Product Life Cycle: Infusion Pump – Premarket Notification [510(k)] Submissions Draft Guidance. April 2010.
15. Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth. 2004;93:212-223.
16. Dolin SJ, Cashman JN. Tolerability of acute postoperative pain management: nausea, vomiting, sedation, pruritis, and urinary retention. Evidence from published data. Br J Anaesth. 2005;95:584-591.
17. Momeni M, Crucitti M, De KM. Patient-controlled analgesia in the management of postoperative pain. Drugs 2006;66:2321-2337.
18. Ratka A, Wittwer E, Baker L, et al. Pharmacokinetics of morphine, morphine-3-glucuronide, and morphine-6-glucuronide in healthy older men and women. Am J Pain Manag. 2004;14:45-55.
19. Sear JW, Hand CW, Moore RA, et al. Studies on morphine disposition: influence of general anaesthesia on plasma concentrations of morphine and its metabolites. Br J Anaesth. 1989;62:22-27.
20. Hutchison RW, Chon EH, Tucker WF Jr, et al. A comparison of a fentanyl, morphine, and hydromorphone patient-controlled intravenous delivery for acute postoperative analgesia: a multicenter study of opioid-induced adverse reactions. Hosp Pharm. 2006;41:659-663.
21. Frost & Sullivan. The New Economics of Healthcare: Evaluating Medical Technologies Based on Actual Value as a Growth Strategy for Healthcare Providers. 2009.
22. White PF. The changing role of non-opioid analgesic techniques in the management of postoperative pain. Anesth Analg. 2005;101(5 suppl):S5-S22.
23. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
24. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137-162.
25. Cheetham TC, Levy G, Niu F, Bixler F. Gastrointestinal safety of nonsteroidal antiinflammatory drugs and selective cyclooxygenase-2 inhibitors in patients on warfarin. Ann Pharmacother. 2009;43(11):1765-1773.
26. Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA. 2006;296(1):87-93.
27. Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev. 2009;(3):CD007076.
28. Moore RA, Wiffen PJ, Derry S, McQuay HJ. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011;(3):CD00793.
29. Mather LE. Opioids: a pharmacologist’s delight! Clin Exp Pharmacol Physiol. 1995;22:833-836.
30. Ved SA, Dubois M, Carron H, et al. Sufentanil and alfentanil pattern of consumption during patient-controlled analgesia: a comparison with morphine. Clin J Pain.1989;5(Suppl 1):S63-70.
31. Clark NJ, Meuleman T, Liu WS, et al. Comparison of sufentanil-N2O and fentanyl- N2O in patients without cardiac disease undergoing general surgery. Anesthesiol.1987;66:130-135.
32. De Castro J. Practical applications and limitations of analgesic anesthesia: a review. Acta Anaesthesiol Belg. 1976;27:107-128.
33. Monk JP, Beresford R, Ward Sufentanil A. A review of its pharmacological properties and therapeutic use. Drugs. 1988;36:286-313.
34. Savoia G, Loreto M, Gravino E. Sufentanil: an overview of its use for acute pain management. Minerva Anestesiol. 2001;67:206-216.
35. Palmer PP, Hamel LG, Skowronski RJ. Single- and repeat-dose pharmacokinetics of sublingual sufentanil NanoTab in healthy volunteers [abstract A1222]. Presented at: the Annual Meeting of the American Society of Anesthesiologists. New Orleans (LA), October 17-21, 2009.
36. Sanford TJ, Smith NT, et al. A comparison of morphine, fentanyl and sufentanil anesthesia for cardiac surgery. Anesth Analg. 1986;65:259-266.
37. Griffin DW, Skowronski RJ, Dasu BN, et al. A phase 2 open-label functionality, safety, and efficacy study of the sufentanil NanoTab PCA System in patients following elective unilateral knee replacement surgery. Poster presented at the 35th Annual Spring Meeting and Workshops of the American Society of Regional Anesthesia and Pain Medicine. Toronto, Ontario, Canada, April 22-25, 2010.
38. Internal AcelRx study data.
39. Minkowitz, HS, Sigla NK, Palmer PP, Combined Analyses of Phase 2 Studies for ARX-01 Sufentanil NanoTab® PCA System for the Management of Moderate to Severe Post-Operative Pain, Poster presented at American Pain Society Annual Meeting, May 2011
40. Minkowitz HS, Skowronski RJ, Palmer PP. A phase 2 multicenter, randomized, placebo-controlled study to evaluate the clinical efficacy, safety, and tolerability of sublingual sufentanil NanoTab in patients following elective unilateral knee replacement surgery. Poster presented at the 35th Annual Spring Meeting and Workshops of the American Society of Regional Anesthesia and Pain Medicine. Toronto, Ontario (Canada), April 22-25, 2010.
41. Singla NK, Skowronski RJ, Palmer PP. A phase 2 multicenter, randomized, placebo-controlled study to evaluate the clinical efficacy, safety, and tolerability 598 Palmer & Miller of sublingual sufentanil NanoTab in patients following major abdominal surgery. Poster presented at the 35th Annual Spring Meeting and Workshops of the American Society of Regional Anesthesia and Pain Medicine. Toronto, Ontario (Canada), April 22-25, 2010.
42. Macintyre PE, Jarvis DA. A Phase 2 Multicenter, Randomized, Placebo-Controlled Study to Evaluate the Clinical Efficacy, Safety, and Tolerability of Sublingual Sufentanil NanoTab in Patients Following Elective Unilateral Knee Replacement Surgery. Pain. 1995;64:357- 364.
43. Kaloul I, Guay J, Côté C, Fallaha M. The posterior lumbar plexus (psoas compartment) block and the three-in-one femoral nerve block provide similar postoperative analgesia after total knee replacement. Can J Anaesth. 2004;51(1):45-51.

Pamela P. Palmer,
MD, PhD,
Chief Medical Officer & Co-Founder
AcelRx
Dr. Palmer has served as Chief Medical Officer and a Board member of AcelRx since she co-founded the company in July 2005. Dr. Palmer has been on faculty at the University of California, San Francisco since 1996 and is currently a Clinical Professor of Anesthesia and Perioperative Care. Dr. Palmer was Director of UCSF PainCARECenter for Advanced Research and Education from 2005 to 2009, and was Medical Director of the UCSF Pain Management Center from 1999 to 2005. Dr. Palmer earned her MD from Stanford University and her PhD from the Stanford Department of Neuroscience.

Larry G. Hamel,
Chief Development Officer
AcelRx
Mr. Hamel has served as Chief Development Officer of AcelRx since September 2006. From 1986 until September 2006, Mr. Hamel served as Product Development Manager, Director Project Management, Executive Director Oral Product Development, and Vice President Oral Products Development at ALZA Corporation. From 1977 until 1985, Mr. Hamel held a number of positions at ALZA Corporation, including Senior Chemist, Research Scientist, and Senior Research Fellow. Mr. Hamel earned is BS in Biology from the University of Michigan.

Badri Dasu,
Chief Engineering Officer
AcelRx
Mr. Dasu has served as Chief Engineering Officer of AcelRx since September 2007. From December 2005 until September 2007, Mr. Dasu served as Vice President of Medical Device Engineering at Anesiva. From March 2002 until December 2005, Mr. Dasu served as Vice President for Manufacturing and Device Development at AlgoRx Pharmaceuticals. From January 2000 until March 2002, Mr. Dasu served as Vice President of Manufacturing and Process Development at PowderJect Pharmaceuticals. Previously, Mr. Dasu served in various capacities in process development at Metrika, and at Cygnus. Mr. Dasu earned his BE in Chemical Engineering from the University of Mangalore, India, and his MS in Chemical Engineering from the University of Tulsa.
Total Page Views: 4906