Issue:June 2018
THERAPEUTIC FOCUS - Antisense Drug Shown to Significantly Reduce Triglyceride Levels in Patients With Severe Hypertriglyceridemia
INTRODUCTION
Hypertriglyceridemia can result from an unhealthy lifestyle, obesity, physical inactivity, excessive alcohol intake, metabolic syndrome, type 2 diabetes mellitus, and more rarely from genetic disorders (eg, familial chylomicronemia syndrome, familial partial lipodystrophy, familial combined hyperlipidemia, familial dysbetalipoproteinemia).
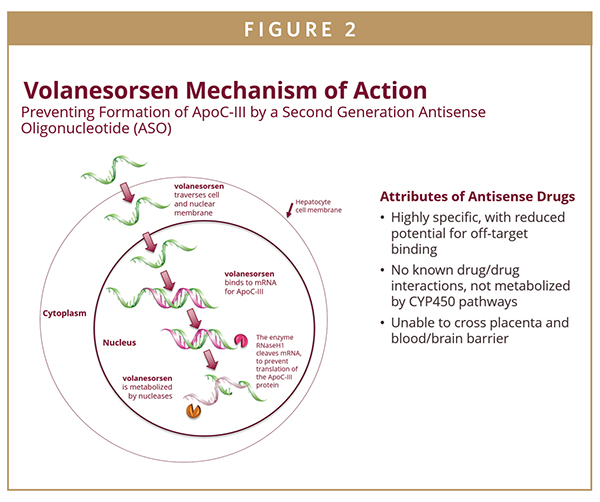
Apolipoprotein C-III (apoC-III) is a key modulator of plasma triglyceride (TG) levels. It has been shown that loss of function mutations in apoC-III are associated with lower TG levels and a reduction in cardiovascular risk while elevated TG levels are associated with increased risk of both cardiovascular events and pancreatitis. In recent years, there has been an effort to develop strategies to reduce levels of apoC-III in order to reduce elevated triglyceride levels. Based on this background, our team led a pivotal study in patients with severe hypertriglyceridemia, defined as triglycerides ≥ 500 mg/dL, with volanesorsen, a second-generation antisense oligonucleotide that inhibits apoC-III synthesis. Antisense technology is based on the use of synthetic nucleic acid sequences to interrupt the production of a specified protein by targeting the corresponding messenger RNA, or mRNA, that encodes that protein. Antisense drugs are therefore able to reduce the level of proteins that cause or contribute to the progression of various diseases, including hypertriglyceridemia.
TARGETING LIPID & LIPOPROTEIN RISK FACTORS
Lipid and lipoproteins, including LDL-C, triglycerides, apoCIII, and Lp(a), are associated with development and progression of atherosclerotic cardiovascular diseases. Moreover, increased TG levels are associated with incidents of acute pancreatitis and liver disease such as non-alcoholic steatohepatitis (NASH). The protein apoC-III, the second most abundant lipoprotein, circulates in the bloodstream attached to particles containing triglycerides and inhibits TG-hydrolysis. High levels of apoC-III have been associated with hypertriglyceredemia.
Mutations in the apoC-III gene that impair its function result in lower TG levels and a correlated reduction in cardiovascular risk. Conversely, overexpression of the same gene has been associated with elevated TG levels and increased risk of both cardiovascular events and pancreatitis. In recent years, there has been an effort to develop strategies to reduce levels of apoC-III in order to reduce elevated triglyceride levels.
THE COMPASS STUDY
The COMPASS clinical trial was a randomized, multi-center, double-blind, placebo-controlled, 26-week, Phase 3 study to evaluate the effect of apoC-III reduction with the investigational antisense drug volanesorsen on TG levels in patients with hypertriglyceridemia. The trial included patients with fasting TG ≥ 500 mg/dL, (n=113, mean ±SD, TG level 1261 ±955mg/dL) who were randomized 2:1 on volanesorsen or placebo. Patients were treated with 300-mg volanesorsen subcutaneously (SC) of placebo once a week for 26 weeks.
Results from the clinical study indicate that antisense technology can result in significant reductions in triglyceride levels in patients with hypertriglyceridemia:
- Patients treated with volanesorsen (n=75) achieved a 71.2% mean reduction in triglycerides after 13 weeks of treatment, compared with a mean reduction of 0.9% in placebo-treated patients (n=38).
- The treatment group included a subset of patients whose hypertriglyceridemia was associated with familial chylomicronemia syndrome (FCS), a rare, genetic disorder characterized by extremely high levels of triglycerides and risk of recurrent, potentially fatal pancreatitis. People with FCS are unable to effectively clear large, triglyceride-rich lipid particles called chylomicrons due to a deficiency of lipoprotein lipase, an enzyme that helps break down triglycerides. In a subset of seven patients with FCS who had average incoming triglyceride level of 2,280 mg/dL, volanesorsen-treated patients (n=5) achieved a mean reduction in triglycerides of 73% from baseline after 13 weeks of treatment, compared with a mean increase of 70% in placebo-treated patients (n=2).
- Results also showed that 82% of patients treated with volanesorsen, including three of the FCS patients, achieved triglyceride levels less than 500 mg/dl after 13 weeks of treatment, compared to 14% of placebo-treated patients (p<0.0001).
- Patients treated with volanesorsen also had reduced risk of pancreatitis events, with no events reported in the treatment group and five reported in the placebo group.
- The most common adverse event in the volanesorsen-treated group of patients was injection site reactions (ISRs), which were mostly mild. In this study with patients who are largely asymptomatic and, unlike FCS patients, do not need to manage the daily burden and symptoms of their disease, 13% of treated patients discontinued due to ISRs and 7% of treated patients discontinued treatment for other non-serious adverse events. There were no deaths in the study. None of the FCS patients in the study discontinued. In addition, there were no serious platelet events in the study. There was one potentially related SAE on the drug-treated arm. This was a report of serum sickness that occurred two weeks after the last study dose and resolved without treatment, and after thorough investigation the sponsor determined that the case was not likely caused by the drug.
The COMPASS study was a supportive study used to further validate findings from the APPROACH study, a randomized, double-blind, Phase 3 study of volanesorsen in the treatment of patients with FCS specifically.
THE APPROACH STUDY
The APPROACH study was a 52-week Phase 3 study in 66 patients with FCS.1-3 While the enzyme lipoprotein lipase (LPL) normally breaks down chylomicrons, people with FCS do not have functioning LPL, resulting in circulating triglyceride levels in the thousands (mg/dL) or more than 10 times the upper limit of normal.1-4 FCS patients have a significant risk of morbidity and mortality, including recurrent episodes of acute pancreatitis, which can be fatal.5-7
In the APPROACH study, FCS patients treated with volanesorsen experienced robust reductions in triglycerides and related benefits, including:
- A 77% mean reduction in triglycerides after three months compared to a mean increase of 18% in placebo-treated patients.
- Volanesorsen-treated patients with the highest documented frequency of pancreatitis attacks suffered no attacks during the 52-week treatment period (p=0.02).
- A reduction in abdominal pain was observed in volanesorsen-treated patients compared to placebo-treated patients.
THE IMPACT ON STANDARD OF CARE
Guidelines established by the Endocrine Society suggest dietary counseling and weight loss for the treatment of mild-to-moderate hypertriglyceridemia in patients who are overweight or obese. Patients with more severe forms of hypertriglyceridemia are typically managed with reduced intake of dietary fat and simple carbohydrates in combination with drug therapy including fibrates, nicotinic acid, omega-3 fatty acids or statins, alone or in combination. In many patients, currently available management strategies are unable to normalize triglyceride levels or decrease the risk of pancreatitis.
Current TG-lowering drugs work mainly through the LPL pathway, thus being largely ineffective in patients with FCS.1,7,8 The results of the COMPASS study provide strong additional support for therapeutic strategies decreasing triglycerides levels by reducing apoC-III concentrations in patients with severe hypertriglyceridemia including the very difficult to treat FCS patient population.
REFERENCES
- Brahm AJ, Hegele RA. Chylomicronaemia–current diagnosis and future therapies. Nat Rev Endocrinol. 2015;11(6):352-362.
- Gaudet D, Brisson D, Tremblay K, et al. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med. 2014;371(23):2200-2206.
- Chait A, Brunzell JD. Chylomicronemia syndrome. Adv Intern Med. 1992;37:249-273.
- Bijvoet SM, Bruin T, Kastelein JJ. The familial hyperchylomicronaemia syndrome. Neth J Med. 1993;42(1-2):36-44.
- Blom D. Hypertriglyceridaemia: aetiology, complications and management. JEMDSA. 2010;15(1):11-17.
- Stuyt PM, Demacker PN, Stalenhoef AF. Pancreatitis induced by oestrogen in a patient with type I hyperlipoproteinaemia. Br Med J (Clin Res Ed). 1986;293(6549):734.
- Tremblay K, Methot J, Brisson D, Gaudet D. Etiology and risk of lactescent plasma and severe hypertriglyceridemia. J Clin Lipidol. 2011;5(1):37-44.
- Williams L, Wilson DP. Editorial commentary: dietary management of familial chylomicronemia syndrome. J Clin Lipidol. 2016;10(3):462-465.
To view this issue and all back issues online, please visit www.drug-dev.com.

Dr. Ioanna Gouni-Berthold is Professor of Medicine at the University of Cologne, where she works as a senior registrar at the Center for Endocrinology, Diabetes, and Preventive Medicine. She is an internist and endocrinologist, double-board certified in the United States and in Germany and a Fellow of the Royal Society of Public Health in the United Kingdom. Her work has been published in various high-impact international journals, such as the JAMA, European Heart Journal, Diabetes Care, and Atherosclerosis.
Total Page Views: 4337