Issue:January/February 2024
SUPPLY CHAIN VISIBILITY - New Tracking Technologies Driving Improvements to Reduce Drug Shortages, Expedite Recalls, Protect & Inform Patients
INTRODUCTION
Supply chain issues continue to plague the healthcare industry, creating delivery delays and product shortages that can interrupt and seriously compromise patient care. Imagine cancer patients, for example, having to stop chemotherapy before the prescribed course of treatment is finished because the necessary drugs are unavailable. If you know someone in this predicament, you’re not alone.
Drug shortages in the US are at an all-time high, with no relief in sight. Solutions are urgently needed to improve supply chain visibility – the ability to “see” where products are located at any point in time during their life cycle – to infuse product stability and help ensure necessary supplies are available when and where they are needed.
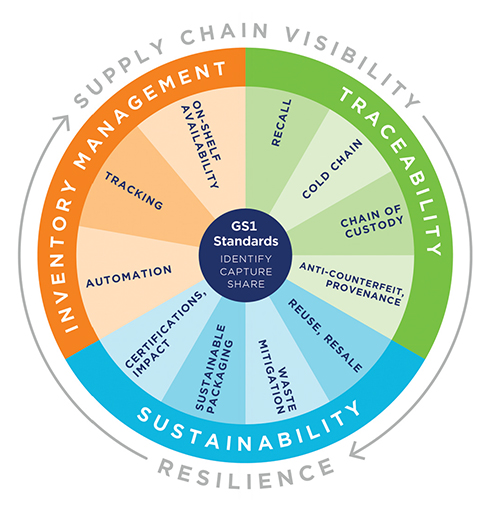
To that end, healthcare industry leaders have been working together to upgrade existing supply chains with long-term, sustainable solutions. New technologies are being leveraged along with industry standards to enable full visibility across the entire supply chain, tracking products all the way from manufacturer to end user. Among them are new, advanced data carriers – capable of holding vast amounts of product information within a tiny footprint on a product label – including two-dimensional (2D) barcodes and improved radio-frequency identification (RFID) tags.
DIGITAL IDENTIFICATION IS THE CORNERSTONE OF TRACEABILITY
Unique product identification is the first building block for track-and-traceability. Encoding a standardized, digital identifier on the product label marks that item and differentiates it from all others. For pharmaceuticals, the identifier is a National Drug Code, embedded in a standardized GS1 Global Trade Item Number® (GTIN®). The US FDA’s Drug Supply Chain Security Act (DSCSA) requires the product identifier plus serial number, lot number, and expiration date to be included in a 2D barcode (eg, GS1 DataMatrix) on unit-level pharmaceutical product packaging. Homogeneous cases must include either a 2D or linear (eg, GS1-128) barcode.
GS1 US, a not-for-profit information standards organization, supports these efforts by providing a system of standards for identifying products, locations, and logistic units, thus enabling supply chain stakeholders like manufacturers, wholesale distributors, and dispensers to meet the DSCSA requirements. The standards-based data enables interoperable, electronic exchange between trading partners, required by the FDA to meet the final DSCSA deadline of November 27, 2023. (The FDA recently announced an enforcement delay until November 27, 2024).
DSCSA is aimed at improving drug traceability and is primarily focused on trace-back to swiftly address issues that could potentially affect patient safety. If a product is suspected to be or deemed illegitimate, understanding the journey of a product at the serialized unit-level enables the identification of vulnerable points within a supply chain where counterfeit drugs can be inserted. Additionally, enabling fast identification and pinpointing the location of products that are recalled, withdrawn, or expired facilitates quick, precise removal from the supply chain to prevent those drugs from being used or sold.
COLLABORATION ACROSS THE SUPPLY CHAIN IS KEY
The supply chain benefits of using 2D barcodes for tracking and tracing can only be realized when all trading partners are aligned on standards and capabilities. Manufacturers are advised to discuss their 2D transition plans with key trading partners, including distributors, wholesalers, third-party logistics providers, healthcare providers, and pharmacies. Potential impact, cost, and benefits of implementing 2D barcodes should be understood for every point where the product is touched – from manufacturing through distribution, storage, and end use.
Optical scanners are needed to automate data capture from the barcode so that all stakeholders can access, ingest, utilize, and share information about the products’ movement all the way through the supply chain. This enables all parties to confirm transactional events (shipping, receiving) and locations to enable real-time visibility – the products can be tracked down to an exact location and status at any point in time by any of the connected trading partners. Locations are identified with Global Location Numbers (GLNs). The record of transactional data detailing the movement of products through the supply chain can be created and shared with the use of GS1’s Electronic Product Code Information Services (EPCIS) standard, as recommended by the FDA for DSCSA.
With such alignment, benefits are also spread across the whole network. From inventory visibility to shipping/receiving awareness, to operational efficiencies and cost savings, and most importantly patient safety – all can be positively impacted by a smoothly run supply chain supported by digitally interoperable, complete records of product manufacture, distribution, and use.
2D BARCODES OFFER PRODUCT TRANSPARENCY ALL THE WAY TO THE PATIENT
Medications are dispensed in healthcare facilities as well as in retail pharmacies and, in the case of OTC drugs, other retail outlets. It makes sense for the traceability mechanisms to also cross channels, and using 2D barcodes makes that possible. Product identification and verification is necessary throughout the entire supply chain for DSCSA even at small, independent pharmacies or individual practitioners who are acting like dispensers, which can be challenging. Industry leaders have provided assistance in the form of assigned GLNs to bring these locations into the traceability information loop.
The benefits do not stop at the hospital or store, however. The utilization of 2D barcodes is further driven by consumers’ growing demand for detailed product data. The expanded capacity of these barcodes enables brands to encode critical product information and to additionally embed a wealth of information, such as ingredients, interactions, contra-indications and side effects, dosing recommendations, and even research results documenting efficacy and outcomes. Links to all that data can be embedded in a 2D barcode and scanned by the consumer with a smartphone. This may be especially useful in pharmaceutical products that are sold at retail. Products could someday even proactively notify users, such as a pill bottle reminding a person to take their daily dose.
Furthermore, the adoption of automatic data capture at the point of care through 2D barcodes streamlines clinical workflows, saving time for clinicians and allowing them to focus on patient care rather than manual data entry into inventory sheets and electronic health records. This technology offers increased accuracy and clinical efficiencies, serving as a valuable cost containment measure for healthcare providers.
NEW & IMPROVED RFID TECHNOLOGY IS GAINING TRACTION
Until recently, RFID has not been considered useful for the healthcare supply chain due to prohibitive costs and technological limitations. But today, new and improved RAIN RFID technology, which uses the UHF band, is generating new attention and a fresh look.
Fast, automated scanning of multiple units, even without a line of sight, can be achieved with RAIN RFID, offering improved efficiency for scanning bulk products in distribution. An RFID reader can scan entire shipments automatically and remotely in a fraction of the time it takes to manually scan individual cases or packages.
Fresenius Kabi, a healthcare company that specializes in life-saving medicines and technologies for infusion, transfusion, and clinical nutrition, became the first pharmaceutical manufacturer to embed medication identification data into an RFID tag in 2020, relying on GS1 Standards to permit full interoperability and compatibility. The company has been using these tags on specified products, including glass and plastic vials and syringes. They reported that using RAIN RFID tracking on medication inventory allows them to scan many drugs at once, more easily track expiry dates, and better maintain tighter inventory levels.
RAIN RFID cannot serve DSCSA requirements on its own; instead, it is considered useful in conjunction with 2D data matrix barcodes. The RAIN RFID tags provide an added layer of protection for pharmaceutical integrity in patient care, while saving healthcare providers time and providing precise inventory control throughout the product’s life cycle.
Using GS1 Standards makes the RFID tags readable anywhere, allowing the industry consistency and delivering on interoperability. To ensure all recipients of RFID-tagged products can decode and understand the product information, healthcare industry stakeholders worked together to identify how manufacturers should encode and provide a “roadmap” to assist the industry in adoption. The Implementation Guideline for RFID in Healthcare Manufacturing: Using GS1 Standards to Enable Visibility and Efficiency document defines the application of GS1 Standards to support adoption of RFID by healthcare supply chain stakeholders. It informs pharmaceutical and medical device manufacturers about how to encode RAIN RFID tags using GS1’s Electronic Product Code (EPC) schemes outlined in the EPC Tag Data Standard (TDS) for automatic data capture to be utilized across the healthcare supply chain.
SUMMARY
Supply chain visibility is crucial in the healthcare industry, perhaps more than in any other marketplace. The ability to know where products are located at any moment in time across the entire supply chain helps all stakeholders improve inventory management, reduce stockouts, and streamline logistics operations. It gives providers and dispensers a chance to make adjustments when necessary, such as finding new alternative sources or substitute products as efficiently as possible. And it enables full traceability for product authentication, provenance, and removal in the case of a recall or product expiration.
The challenges of today’s evolving markets and healthcare infrastructure demand new, digitally interconnected solutions that can be shared across increasingly complex supply chains. Two-dimensional barcodes offer wide-ranging benefits and are needed to satisfy the traceability requirements of DSCSA, while new and improved RAIN RFID technology holds promise for operational efficiencies that can help pave the way to faster, more reliable delivery of drugs, helping maintain the integrity of our healthcare systems.

Tracy Nasarenko, Senior Director of Community Engagement at GS1 US, is an accomplished leader with more than 20 years of experience in healthcare and pharmaceutical supply chain, finance, product management, marketing, and operations. In her current role, she leads a collaborative pharmaceutical industry group focused on addressing supply chain challenges and meeting the requirement of the Drug Supply Chain Security Act (DSCSA). Using GS1 Standards, the most widely used supply chain standards in the world, she guides the implementation of these standards to help the pharmaceutical industry deliver safe products to patients. Prior to joining GS1 US, she held several leadership positions with SPI Pharma Inc. Most recently, she served as a site general manager, overseeing end-to-end operations and performance of the entire team, where she built a culture of engagement, innovation, and collaboration. She earned her Bachelor’s degree from Villanova University and her MBA from West Chester University.
Total Page Views: 4670










