Issue:Respiratory e-Book 2020
2020 Respiratory Drug Development eBook – Inhaled Medications for Treating Unconventional Respiratory Diseases
The global spread of COVID-19 put respirators and ventilators at the center of almost every news cycle. Reports of their limited supply became a chief concern for healthcare workers. In March 2020, Johns Hopkins University estimated the demand for ventilators to be as high as 1 million in the US alone, with only 160,000 being available.1 As government and researchers navigate the solution for the COVID-19 outbreak, experts predict the demand for respiratory care devices will be inelastic.
According to the current analysis of Reports and Data, the global Respiratory Care Devices Market was valued at $18.41 billion in 2019 and is expected to reach $37.04 billion by 2027.1 Three years ago, the World Health Organization considered respiratory diseases to be the leading causes of death and disability in the world. Nearly 65 million people suffer from chronic obstructive pulmonary disease (COPD), and 3 million are expected to die from it each year, making it the third leading cause of death across the world. Moreover, approximately 334 million people suffer from asthma, affecting 14% of all children globally.1
Inhaled therapies can be further enhanced by next-generation devices. In fact, the global smart inhalers market is set to gain impetus from the rising utilization of digital technology in the field of healthcare devices. It is aiding in increasing the efficiency of drug delivery devices and is, in turn, speeding up the treatment procedures. The smart inhalers market size was $28.62 million in 2019 and is projected to reach $445.40 million by 2027.2
This market is segmented into metered dose inhalers (MDIs) and dry powder inhalers (DPIs). Of these, the MDI-based smart inhalers are set to gain more market share in the coming years because they aid in controlling and monitoring the amount of drug to be delivered per a patient’s requirements.
Inhalation devices are also being used to treat unconventional diseases. For instance, a biopharmaceutical company is in late-stage clinical research for an inhaled insulin for diabetes. And there are others studying the nose-to-brain route as an alternative to the blood brain barrier to treat neurological diseases like Alzheimer’s and Parkinson’s disease. Additionally, inhaled drug formulations are proving effective in treating migraines because of their rapid absorption.
This second annual Drug Development & Delivery Respiratory eBook focuses on a few companies that are taking inhalation devices to a new level in drug delivery.
References
- Respiratory Care Devices Market To Reach USD 37.04 Billion By 2027, Reports and Data, April 2, 2020, https://www.globenewswire.com/newsrelease/2020/04/02/2011057/0/en/Respiratory-Care-Devices-Market-To-Reach-USD-37-04-Billion-By-2027-Reports-and-Data.html.
- Smart Inhalers Market to Exhibit 41.1% CAGR till 2027; Higher Usage of Digital Technology in the Drug Delivery Devices to Skyrocket Demand, Fortune Business Insights, April 30, 2020, https://www.globenewswire.com/news-release/2020/04/30/2025572/0/en/Smart-Inhalers-Market-to-Exhibit-41-1-CAGR-till-2027-Higher-Usage-of-Digital-Technology-in-the-Drug-Delivery-Devices-to-Skyrocket-Demand-says-Fortune-Business-Insights.html.
![]()
A Focus on Inhalation: Integrated Product Development Services
 By: Richard Harper, Vice President of Marketing, Vectura
By: Richard Harper, Vice President of Marketing, Vectura
Vectura integrates formulation, device, and development capabilities to offer a broad range of services to help customers bring inhaled medicines to market. With more than 20 years of experience focused on inhaled product development, the company’s track record offers customers confidence in Vectura as a trusted development partner; since 2012, Vectura’s formulation and device technology have contributed to the success of 11 inhaled medicines, launched by partners and licensees, generating $10 billion in sales and used by 9 million patients worldwide.1
From Pre-clinical Development to Commercial Scale-up
Vectura offers comprehensive services from the pre-clinical stage of development, when customers are looking to overcome complex inhaled formulation challenges, select a delivery device, and move quickly to the clinic. With expertise in powder and liquid formulations, as well as a portfolio of proprietary device and formulation technologies, Vectura can help customers developing a wide range of small molecules, biologics, and complex combinations.
As products progress through the clinical development phases, customers require greater dosing flexibility, and confidence in process scalability, as well as robust, commercial-ready delivery devices. Vectura’s integrated approach ensures these key considerations are designed into a customer’s program, allowing seamless scale-up from laboratory to pilot scale and through to commercial scale, enabling effective transfer to commercial manufacturing.
Being able to provide customers with a single partner for formulation and device expertise from early development to late-phase clinical trials and beyond offers real benefits in terms of continuity, shortened timelines, and reduced costs.
The Right Delivery Device?
No two development programs are the same, and many factors influence the selection of the most appropriate device, including the development stage, technical considerations, and patient/disease-state characteristics. In early development, a unit-dose capsule or blister device, or a nebulizer, may offer benefits in terms of speed and cost, while a multi-dose dry powder inhaler (DPI), pressurized metered dose inhaler (pMDI) or nebulizer may be required as a commercial-ready platform to support a later-phase program.
Vectura has development expertise across a range of technology platforms, including unit-dose and multi-unit dose DPIs, pMDIs, and smart nebulizers. This allows a “device agnostic” approach to best meet the needs of the customer, their development program, and ultimately, the patient. Vectura’s proprietary DPIs are derived from a commercially validated platform, with a range of user interfaces and payload volumes, giving customers confidence of performance and a proven regulatory track record.
The company’s breath-actuated nebulizers with guided inhalation have been developed to improve lung delivery, with the aim of achieving better clinical outcomes and/or shortened treatment times.
pMDIs remain an important option for many patients, and all the device components have important roles in determining characteristics of the aerosol and drug delivery to the lung. Vectura’s extensive expertise in pMDI development can help customers developing a new product or optimizing the formulation and device performance of an existing product.
Supporting Development Success
With extensive analytical testing facilities and equipment, Vectura’s large, experienced group of analytical scientists can undertake the comprehensive array of test methodologies and physical properties characterization required for inhaled products, as well as stability studies and device verification testing.
Crucial to any inhaled program’s success is navigating the complex regulatory requirements. Vectura’s medical, regulatory, device vigilance, and pharmacovigilance teams can support programs and help ensure the smoothest path to product approval. Vectura offers particular expertise in global regulatory strategies and submissions for both new chemical entities and generics, as well as providing regulatory support for medical devices and combination products.
The Next Generation of Inhaled Medicines
Inhalation continues to be an important route of administration for both respiratory and non-respiratory product development. Optimizing drug formulation and delivery device development can unlock the potential for innovative inhaled medicines that can revolutionize patients’ lives. Vectura is at the forefront of integrated drug and device development, and helping customers to deliver the inhaled therapies of the future.
If you would like to find out how your inhaled development program could progress with Vectura’s capabilities, contact enquiries@vectura.com.
Reference
1. Evaluate Pharma; internal estimates, 2018.
Responding to the Latest Trends in Inhalation Drug Development With Contract Services
 By: Shabbir Mostafa, Global Key Account Director, Recipharm Inhalations
By: Shabbir Mostafa, Global Key Account Director, Recipharm Inhalations
Over the last decade, the inhalation drug market has grown at a rapid rate. The rising cases of respiratory diseases across the world, including asthma and chronic obstructive pulmonary disease (COPD), have acted as catalysts for this, along with the growing acceptance of inhalation as a drug delivery method with many benefits. By delivering therapeutic concentrations directly to the site of treatment in the lung, inhaled medicines can provide a fast onset of action while limiting systemic exposure. This also means that smaller doses are possible, minimizing the risk of undesirable side effects for patients, while still achieving high efficacy.
Expanding Beyond Respiratory Diseases
Inhaled medicines have been a vital source of treatment for respiratory diseases for more than 60 years. However, inhalation dosage forms are now being explored for other therapeutic areas. This includes the treatment of diabetes by delivering insulin via the lung, and for hereditary conditions including cystic fibrosis, supporting pain relief. Similarly, drugs delivered nasally have the potential to treat neurological disorders such as Alzheimer’s and Parkinson’s disease, offering more direct access to the central nervous system.
The changes in the potential application of inhalation treatments have created new opportunities. To encourage innovation, the industry has seen the introduction of favorable approval routes, including the 505(b)(2) regulatory pathway to support reformulation work of existing medications.
Eliminating Environmental Concerns
In addition, there is increasing pressure to develop more environmentally responsible industry practices, and this has undoubtedly impacted the respiratory healthcare sectors where pressurized metered dose inhalers (pMDIs) are one of the most prominent delivery methods for inhalation treatments. As other industries move away from the use of propellants that have a negative environmental impact, the pharma industry must also explore alternatives to ensure the continued supply of vital medicines. From a patient compliance perspective, it is vital to sustain supply of pMDIs, since not all patients are able to use alternatives, such as dry powder inhalers (DPIs), because of their compromised lung function or breathing capability.
Recipharm Inhalation SolutionsTM Responds to These Challenges
All these factors have created many challenges for pharmaceutical companies to overcome. As a leading contract development and manufacturing organization (CDMO), Recipharm helps to manage this complexity, supporting development and manufacturing efforts for inhalation products, including MDIs, DPIs, and nasal sprays.
In 2019, we launched Recipharm Inhalation Solutions™ with the aim of providing customers with an integrated service from development through to commercialization. Our offering also includes dedicated analytical services for inhalation products, including comprehensive extractable and leachable (E&L) expertise.
Every day, our team contributes to advances in inhalation science, helping our customers to launch new patient-centric products that improve the lives of people living with prevalent respiratory diseases, as well as other disease areas.
Establishing a Leading Inhalation Company & Top-5 CDMO
To meet growing demand for inhalation drug products, Recipharm has invested in its capabilities and capacities in recent years, complementing the inhalation development expertise of its team in Research Triangle Park, North Carolina.
In 2018, the CDMO acquired Sanofi’s former inhalation product manufacturing facility in Holmes Chapel, UK. This provided us with specialist technologies for MDIs and nasal sprays as well as state-of-the-art equipment for the scale-up and commercial manufacture of inhalation products.
In February 2020, Recipharm then acquired Consort Medical and this has played a huge part in strengthening our inhalation offering. With the addition of Consort’s highly successful Bespak brand, we are now a leading inhalation contract development and manufacturing company with inhalation device capability.
Recipharm is now able to handle projects from small- to large-scale production. The team has the technical ability to work with different devices and combination products with multiple APIs. Our end-to-end offering provides customers with a truly seamless outsourcing service for inhalation products.
For more information, please visit https://www.recipharm.com/solutions/recipharm-inhalationsolutions.
Novel In Vitro Techniques Demonstrate Bioequivalence & Accelerate Orally- Inhaled & Nasal Drug Product Development
 By: Ian Flaherty, Senior Product Manager, Proveris Scientific
By: Ian Flaherty, Senior Product Manager, Proveris Scientific
In 2012, the U.S. FDA started the Generic Drug User Fee Amendments (GDUFA) program, aimed at expediting the delivery of safe and effective generic drugs to the public and improving upon the predictability of the review process.1 In the interim, numerous product-specific guidance documents have been released for orally-inhaled and nasal drug products. In May 2019, the FDA released a product-specific guidance for beclomethasone dipropionate2 that introduced approaches using new, alternative in vitro characterization studies that are more representative and/or predictive of the clinical effect at the local sites of action in the human respiratory tract. Characterization of the emitted aerosol spray velocity profiles and evaporation rate were proposed as alternative tools for use in comparative clinical endpoint bioequivalence (BE) studies. Also, it was encouraged to apply human-realistic mouth/throat models as well as breathing profiles to existing in vitro testing.

Proveris® Laboratories offers Orally-Inhaled and Nasal Drug Product Development (OINDP) expertise to pharmaceutical developers, including contract services employing the new innovative in vitro techniques outlined by the FDA. These include measuring spray velocity, evaporate rate, and quantifying deposition of inhaled drug products using human-realistic models.
Spray Velocity
A method to measure plume front velocity based on the well-established Proveris SprayVIEW® technique (which produces calibrated, time-synchronized image sequences of the entire aerosol plume and duration) was developed by Proveris scientists.3 The study shows a straightforward data analysis method based on the current plume geometry (PG) measurements to generate a plot of distance travelled by the aerosol plume front versus time, thereby calculating the velocity. This method has the sensitivity to distinguish plume front velocity differences between different types of products, propellants, and formulations.
Evaporation Rate
The evaporation fraction approach was also developed based on the SprayVIEW technique (published in the RDD 2020 conference proceedings4). This method integrates the dynamic spray pattern (SP) data and correlates the cumulative image intensity results with the drug product mass (formulation + propellant). The technique then allows for a comparison of the drug product mass loss to the baseline, demonstrating the percentage loss of mass due to evaporation (evaporation fraction) at various distances from the inhaler mouthpiece edge.
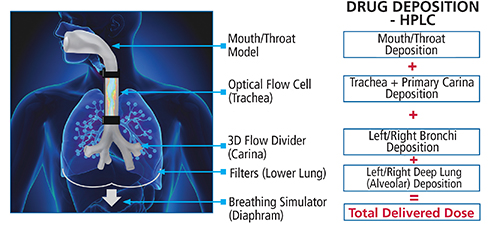
Schematic of Proveris Scientific’s Novel INVIDA (in vitro inhaled drug analysis) Platform.
Quantifying Deposition Using Human-Realistic Models
In addition, Proveris has developed a novel platform called INVIDATM with emphasis on quantifying the regional deposition of aerosol drug products within different regions of the human respiratory tract. Use of a programmable breathing module allows human-realistic breathing profiles for normal and diseased states to be evaluated. Being fully programmable allows patterns published in the literature to serve as a baseline, as well as patterns created de-novo to optimize studies. Visual mapping along with quantitative measurements enables researchers to better understand their product’s performance. Trending critical factors and measurements enable scientists to better predict in vivo performance.
The performance of OINDPs is affected by a combination of formulation, device, and patient usage. Employing alternative in vitro approaches that are human-realistic can enable companies to make data-driven decisions and expedite product development and approval while saving time and resources. Proveris Scientific’s INVIDA platform, along with novel techniques for characterizing plume front velocity and evaporation fraction, is built to help minimize the gap between in vitro and in vivo performance to predict true product performance and ultimately reach product approval. Study results utilizing these techniques are available on the Proveris website at https://www.proveris.com/scientific-posters/.
References
- FDA: Generic Drug User Fee Amendments [https://www fda.gov/industry/fda-user-fee-programs/generic-druguser-fee-amendments]. Accessed October 31, 2019.
- FDA Beclomethasone dipropionate Inhalation Aerosol Metered NDA 207921 PSG Page RC May 2019.
- Liao, L., Ramos, K. & Farina, D. (2019). A Novel Characterization of Emitted Aerosol Velocity Profiles from Metered Dose and Soft Mist Inhalers (pMDI and SMI). In Drug Delivery to the Lungs Proceedings (Vol. 30, 2019:287–290.
- Edinburgh, UK: Aerosol Society. Liao, L., Sule, A., Okorodudu, B., Flaherty, I., Chauhan, H., Newman, A., Sule, S., Turner, R. (2020). The Effect of Ethanol Concentration on pMDI Evaporation Fraction. In Respiratory Drug Delivery Proceedings. Volume 2, 2020: 429-432.
Total Page Views: 8765









