Issue:April 2018
PREFILLED SYRINGES - Prefilled Syringe Automated Inspection & End-Product Testing
INTRODUCTION
Prefilled syringes are used for many therapeutic biologic formulations, and are a good option for ophthalmic injections that require very low volumes of the drug product.1 They are useful for biologics and other expensive drug products because the overfill volume for the syringe is much lower than the volume required for products filled into vials. Another advantage of a prefilled syringe is their ease of use. They are suitable for self-delivery by the patient and reduce the risk of contamination when compared to the multiple steps required for withdrawing a dose from a vial. They are, however, challenging to manufacture with respect to filling, sealing, inspecting, and conducting testing that is specific to the primary packaging. Challenging operations for prefilled syringes that are not often discussed in the literature include automatic inspection and testing specific to the final product. Filled dosage units intended for parenteral administration must be inspected for visible particulates to the extent possible so that they are considered essentially free of particle contamination.2-5 The filled units may undergo 100% manual inspection, semi-automated inspection, or fully automated inspection. There are specific requirements for conducting the inspection and the minimum intensity of light used for the inspection.5 These requirements also apply when using fully automated inspection equipment.
There are additional requirements for end-product testing that are specific for prefilled syringes. For example, functionality tests are conducted to ensure proper movement of the plunger within the syringe barrel.6,7 Container closure integrity testing is conducted to ensure there are no leaks that could affect the sterility assurance of the product.8-11 The numerous requirements for product inspection and evaluation require expertise in working with the equipment as well as knowledge of the available methods for evaluation. This article introduces the common equipment available for automated inspection and discusses inspection testing methods for prefilled syringes.
AUTOMATED INSPECTION
Different companies offer a variety of semi-automated and fully automated inspection equipment (Table 1). This provides many choices for companies in need of inspection equipment. The fully automated inspection equipment operates using a vision detection system. Most are based on the static division system in which there is a light source in front of the syringe and a diode array detector located behind the syringe. The software tracks a voltage drop across a shadow that could be indicative of a particle or a defect. Syringes enter the machine and pass through two different carousels. One carousel spins the syringe or vial to create a vortex in the solution, and the second carousel completes the inspection (Figure 1). The syringes enter a carousel and are held in place using tooling specifically designed for the size and type of syringe being inspected. Cosmetic defects and particle inspections are conducted on a single carousel equipped with two different inspection stations.
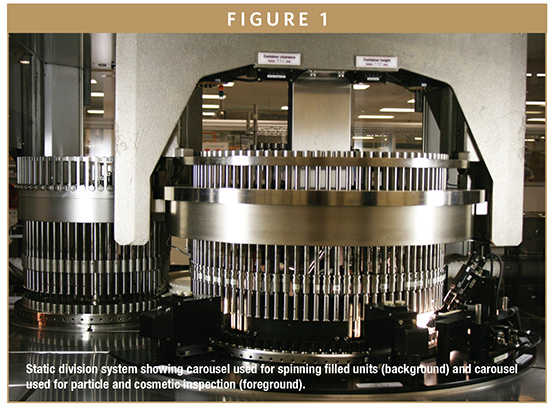
Syringes are inspected for particles after they are spun to create a vortex on the initial carousel. The vortex should cover the entire product-contact surface of the syringe barrel. The goal is to bring any particle that may be in the solution to the surface of the contents of the syringe and to do so without creating bubbles or foam. Particles will be detected if they block light transmitted through the syringe.12 The challenge is that particles may not be detected if they are attached to the surface or ribs of the plunger or located within the cone of the syringe near the fluid path. Fortunately, it is rare to find particles located on the plunger or in the cone of the syringe, and they are often found by visual observation. Syringes that contain particles or fibers adhered to the surface of the syringe barrel will be rejected as cosmetic defects. Syringes that are rejected by the equipment are manually inspected to determine if they are truly a cosmetic defect or if foreign material is present in the syringe. An investigation may be initiated if rejected syringes appear with foreign material. The syringes are examined for cosmetic defects while on the same carousel immediately after inspection for particles.
Cosmetic defects are detected using a high-resolution camera and an image subtraction algorithm. The equipment is calibrated using syringes from a defect library. The defect library contains syringes exhibiting specific defects observed at the manufacturing site. These may include syringes with cracks or inclusions in the glass. They can also include syringes with cracked flanges, but many other possible defects exist. The defect samples are later used to challenge the equipment before and after inspection of each batch to ensure that the defects are identified by the equipment. The goal is for no more than one defect to be accepted. The set of cosmetic defects is based on the acceptable quality limit (AQL). The goal is to ensure that the number of false rejects does not exceed an established threshold percentage of the total defective units identified. Depending on the established threshold, the equipment would, for example, identify 98% of the defective units and have no more than 2% as false rejects. The equipment is qualified by introducing 10 defective units to the equipment along with 10 acceptable units. The number of acceptable units included for a test is increased after each successful inspection until 10 defective units can be identified in a batch of approximately 10,000 acceptable units. The process is labor intensive, requires close communication with customer support services for the equipment, and must be completed for each new product and different size or type of prefilled syringe. The syringes that pass automated inspection may proceed to automated container closure integrity testing (CCIT). Most equipment available for automated visual inspection can also be equipped with automated CCIT, such as high-voltage leak detection systems.
CONTAINER CLOSURE INTEGRITY TESTING (CCIT)
Minute cracks, pinholes, and needles that pierce the endcaps can be missed during the inspection process for prefilled syringes. The only way to ensure there is no risk to sterility assurance of the filled syringes is to test each one. Testing each filled unit was not possible before non-destructive, fully automatic testing instruments were invented. A variety of CCIT methods are now available. Chapter 1201.2 of the USP defines the methods as deterministic and probabilistic.9 Deterministic methods are preferable and consist of non-destructive methods of testing making it possible to test each unit that was manufactured. Examples of the deterministic methods include vacuum/pressure decay testing, high-voltage leak detection, and methods that utilize a laser to analyze the head space of a filled unit. There is also an instrument by HeuftTM called the Syringer® that uses x-ray pulses to identify syringes with bent needles and needles that puncture the caps. Vacuum/pressure decay, headspace analysis, and high-voltage leak detection methods are more common and are easily attached to a production line for 100% inspection (Figure 2).

The vacuum/pressure decay methods subject individual units to a preset vacuum or pressure and monitor for changes in the vacuum/pressure that can indicate a leak. The data can also be used to calculate the size of the leak. The method can be combined with a mass flow recorder to monitor the loss of headspace gas. This method is also referred to as a mass extraction method.
A laser can be used to examine the headspace of a filled unit using wave modulated near infrared spectroscopy or frequency modulated near infrared spectroscopy. The laser is directed through the headspace of the unit, and the data are compared against a set of standards. The standards are prepared based on the gas or on levels of water vapor that are being measured.
Most of the probabilistic methods for testing container closure integrity are destructive methods. They include bubble emission, microbial challenge, and tracer liquid testing.9 The tracer gas detection method is a non-destructive probabilistic method. This method is typically used for packaging in which the package is exposed to helium for a certain amount of time. The package is removed and tested for the presence of helium that may be flowing from a leak in the packaging.
The bubble emission, microbial challenge, and tracer liquid test methods are conducted by immersing the filled units in water, in a concentrated bacterial suspension, or in a liquid that can be used as a tracer, such as a dye or metallic ions. Pressure is applied to the immersed units to provide a challenge. The units are examined for the emission of bubbles from a possible leak, microbial growth after incubation, or a color change or detection of the metal ions, respectively. The destructive nature of the tests prevents them from being used to test each unit. Samples are obtained randomly and tested together using one of the methods. This is typically conducted initially after manufacturing and then tested over time as part of a stability study.
FUNCTIONAL TESTING
Testing the performance of a prefilled syringe after filling and throughout the shelf-life is recommended.13 Part of this testing includes glide force and break force testing. The break force is the energy required to initiate movement of the plunger in the syringe barrel. The glide force is the energy required for the plunger to continuously move through the barrel of the syringe. It can be uncomfortable for the person delivering the dose and for the patient if excessive force is required to initiate movement of the plunger. Continuous, smooth, movement of the plunger through the barrel is desired. Incomplete coverage of the barrel with silicone oil or redistribution of the oil can cause the plunger to intermittently stop traveling the length of the barrel. This is referred to as “chattering” and can also be uncomfortable. Instruments for testing the break force and glide force are available and include instruments from Zwick/RoellTM and InstronTM.
SUMMARY
The shape of prefilled syringes and the small volumes they contain make them challenging to inspect for particles and cosmetic defects. Automated inspection systems greatly reduce the time needed for inspection and improve the detection of defects. The inspection systems can often be combined with automated container closure integrity testers to ensure sterility of the entire batch. Expertise is needed for qualification and validation of the inspection equipment. Specific inspection criteria must be entered for each type of syringe inspected on the equipment. In addition, a library containing syringes with common defects is needed for qualification and testing of the equipment.
Prefilled syringes are routinely examined for functional performance as well as container closure integrity. Instruments are available for testing the force needed to initiate movement of the plunger and the force needed for movement of the plunger through the syringe barrel. The tests are often included in stability studies to ensure proper performance of the prefilled syringe over time.
ACKNOWLEDGMENT
The author would like to thank Jason Hasler, Senior Validation Specialist, Baxter’s Bloomington, IN, facility, for his contributions and knowledge of the inspection equipment.
REFERENCES
1. Sacha G, Rogers A, Miller RL. Pre-Filled Syringes: A Review of the History, Manufacturing, and Challenges. Pharmaceutical Development and Technology. 2015;20(1):1-11.
2. The United States Pharmacopeial Convention. General Requirements Injections and Implanted Drug Products (Parenterals) – Product Quality Tests. USP 40 – NF 35;2017.
3. The United States Pharmacopeial Convention. Physical Tests/Subvisible Particulate Matter. USP 40 – NF 35; 2017.
4. The United States Pharmacopeial Convention. Physical Tests / Particulate Matter in Injections. USP 40 – NF 35; 2017.
5. The United States Pharmacopeial Convention. Physical Tests/Visible Particulates in Injections. USP 40 – NF 35; 2017.
6. EMEA, ICH Q6A. Note for Guidance Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances (CPMP/ICH/367/96).
7. FDA, CDER. Guidance for Industry Container Closure System for Packaging Human Drugs and Biologics.
8. USP 40 – NF 35 Errata to Second Supplement. Package Integrity Testing in the Product Life Cycle – Test Method Selection and Validation.
9. USP 40 – NF 35 Errata to Second Supplement. Package Integrity Leak Test Technologies.
10. USP 40 – NF 35 Errata to Second Supplement. Package Seal Quality Test Technologies.
11. USP 40 – NF35 Errata to Second Supplement. Package Integrity Evaluation – Sterile Products.
12. Baczewski J. Flexible Inspection of Prefilled Syringes. ONdrugDelivery. October 2015(61):35-37.
13. U.S. Department of Health and Human Services, Food and Drug Administration. Glass Syringes for Delivering Drug and Biological Products: Technical Information to Supplement International Organization for Standardization (ISO) Standard 11040-4.

Dr. Gregory A. Sacha is a Senior Research Scientist in Research and Development at Baxter BioPharma Solutions with 18 years of experience in the pharmaceutical industry. Dr. Sacha currently leads a group of scientists in formulation and process development for parenteral products with a focus on the formulation of large molecules and lyophilization. His research interests include formulation variables affecting the stability of large molecules, thermal analysis of pharmaceutical formulations, and factors affecting scale-up of lyophilization processes. Dr. Sacha’s list of publications includes topics in parenteral product packaging and prefilled syringes.
Total Page Views: 12598









