Issue:November/December 2020
POLYMERIC DELIVERY SYSTEM - Next-Generation Long-Acting Implantables Using Surface-Eroding Elastomers
ABSTRACT
Poly(glycerol sebacate) (PGS) (Regenerez®, Secant Group) is a synthetic polyol resin that can be cross-linked using urethane chemistry to create poly(glycerol sebacate) urethane (PGSU) (Hydralese™, Secant Group). PGSU is a highly flexible, water-impermeable, shelf-life stable, and biocompatible elastomer that biodegrades via surface erosion. Unlike bulk-degrading polymers or non-degradable polymers that rely on diffusion, the hydrolytic surface erosion properties of PGSU confer near zero-order release kinetics, even at high-drug loadings, and maintains a near-constant release rate across drug loadings. PGSU is an attractive delivery system for very hydrophobic drugs, which otherwise may not be able to diffuse out of the matrix, and for very hydrophilic drugs, which otherwise may have an uncontrolled burst release. PGSU can be loaded with both hydrophilic and hydrophobic drugs up to 80% w/w, demonstrating minimal burst release in vitro and in vivo and sustaining release for greater than 6 months’ duration. The cross-linking density of PGSU is critical to reducing fluid percolation in and API permeation out of the matrix, especially at high-drug loadings, in order to limit burst release and diffusion. PGSU ultimately offers many advantages over other polymers for long-acting implantables (LAIs), particularly for high-loading, long-duration implants that are gaining interest in the pharmaceutical industry.
THE NEED FOR AN IMPLANTABLE DRUG DELIVERY SYSTEM
Long-acting implant technology is taking center stage in pharmaceutical development. This technology is gaining traction because it allows a sustained delivery of a therapeutic drug over the course of many months and even years, improving patient compliance and comfort. Long-acting implants can also deliver drugs systemically or to a targeted site, avoid the inefficiencies of first-pass metabolism, and reduce side effects.
One of the biggest advantages of implantable drug delivery systems, whether manufactured as rods, tubes, sheets, fibers, microspheres, or coatings, is that they can be made of bioresorbable polymers. Over time, the body breaks down and metabolizes these polymers until there is no implant left to remove at the end of drug therapy.
LIMITATIONS OF CURRENT POLYMERS ON THE MARKET
Commercially available bulk-degrading polymers, such as poly(lactic-co-glycolic acid) (PLGA), polyglycolic acid (PGA), polylactic acid (PLA), polycaprolactone (PCL), and non-degradable polymers, such as poly(ethylene-co-vinyl acetate) (EVA), polyurethane (PU), and silicone, demonstrate many limitations as drug delivery systems. Most notably, these types of polymers rely on diffusion for drug delivery; therefore, achieving high-drug loading with sustained release is challenging due to the steep concentration gradient that drives fast diffusion. With such polymers, this dose-dependent release rate is sometimes sufficient for achieving a few months of controlled release when the loading is about 40% w/w or less. Higher loadings exhibit significantly faster release rates and often only provide 1 month of controlled-release therapy.1 These polymers can also exhibit a severe increase in stiffness at higher drug loadings, impacting patient comfort and leading to brittle fracture. Moreover, while degradation of the delivery vehicle is generally considered a market preference, bulk-degrading polymers exhibit inconsistent mass-loss behavior and, consequently, uncontrolled release. In contrast, surface-eroding polymers display a controlled and predictable volume loss over time, occurring from the outside edge slowly inward, allowing zero-order release kinetics and a dose-independent release rate.
Highly soluble active pharmaceutical ingredients (APIs) pose a challenge to non-degradable and bulk-degradable polymers because they are likely to rapidly diffuse away from the polymer matrix, causing a large burst release and fast release rate. On the other hand, poorly soluble APIs pose a challenge to non-degradable and bulk-degradable polymers because these APIs have a difficult time diffusing away from the polymer matrix, causing insufficient release following implantation. A polymeric delivery system that does not rely on diffusion and releases both highly soluble and poorly soluble APIs agnostically through surface erosion addresses an unmet market need.
CHEMISTRY OF POLY(GLYCEROL SEBACATE) URETHANE (PGSU)
Poly(glycerol sebacate) (PGS) (Regenerez, Secant Group) is a polyester prepolymer resin synthesized by reacting glycerol and sebacic acid together in a polycondensation reaction. Secant Group’s water-mediated reaction process to synthesize PGS conveys many product and manufacturing benefits.2 PGS resin can be further cross-linked into a solid elastomer thermoset, poly(glycerol sebacate) urethane (PGSU) (Hydralese™, Secant Group), which is formed using urethane chemistry by reacting the PGS resin polyol with an isocyanate cross-linker and a tin-based catalyst. API is incorporated by blending the neat powder with PGS resin prior to urethane reaction. This PGSU reaction occurs within minutes at room temperature, allowing inclusion of thermolabile and form-sensitive APIs in the formulation; the process may be accelerated using mild heat. The total amount of tin catalyst used is well below the human permitted daily exposure limit, and no residual isocyanate is leftover after reaction completion. The end result is a drug-loaded, elastomeric PGSU implant (Figure 1).
PGS resin may be formulated solvent-free, or it can be solvated to reduce viscosity using USP class 3 solvents, such as acetone and propyl acetate. If solvents are used, residual solvent can be driven off using vacuum and mild heat at 40°C. Solvent-free and room-temperature manufacturing reduces post-processing steps and avoids detrimental changes to the API.
The mass ratio of PGS:hexamethylene diisocyanate (HDI), which can be converted to isocyanate:hydroxyl stoichiometric ratio if the polyol’s hydroxyl value is known, dictates the cross-linking density obtained with PGSU (Figure 2).
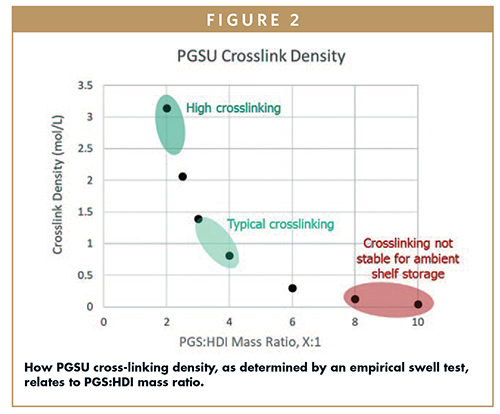
Selection of an appropriate PGSU cross-linking provides a shelf-stable product that can be stored at room temperature and humidity for up to 3 years, per ICH Q1A(R2) long-term and accelerated studies. By tuning the cross-linking density, PGSU matrix permeability, elasticity, degradation, and drug release can be manipulated to desired specifications that meet the target product profile.3
The two-component PGSU reaction is thoroughly mixed within its pot life to achieve API content uniformity and cross-linking uniformity. High-shear mixing, achieved using speed mixers, static mixers, dynamic mixers, twin-screw compounders, or similar equipment, evenly distributes and fully incorporates both API and isocyanate into the PGS resin. One solution for fabricating PGSU implantable rods uses a dual-feed system, providing consistent and scalable manufacture. Dual-feed techniques can be scaled up and translated to a reaction injection molding manufacturing process to support global supply needs. Ultimately, PGSU and API-loaded PGSU can be manufactured into rods, tubes, sheets, fibers, microspheres, coatings, and other geometries by a variety of processing techniques, such as reaction injection molding, extrusion, casting, drawing, emulsion, spray coating, dip coating, and additive manufacturing. PGSU can be gamma irradiated as a method of sterilization without any detrimental effects to product performance.
Once implanted, PGSU is biodegradable via surface erosion, making it an excellent candidate for controlled drug release. The mechanism of surface erosion in vivo for PGSU is hydrolysis, enzymatic degradation by esterase and lipase, and oxidative degradation. By altering the cross-linking density of PGSU and stoichiometry of the starting reagent PGS resin, the elastomer’s degradation rate can be tailored to match the desired duration of drug treatment. Degradation rates of 2 to 12 months are easily achievable with PGSU, spurring opportunities for many long-acting therapies.
THE ELASTICITY ADVANTAGE
As a bioresorbable urethane with tunable degradation rates, PGSU is designed to deliver API in a controlled, surface-eroding mechanism. It also provides elastomeric compliance that can be tailored to mimic the elastic properties of tissue. These properties of PGSU present a stark contrast to rigid, thermoplastic lactides and glycolides that bulk degrade and lack sufficient elasticity. For example, when PLGA and PCL are formulated with high-drug loadings, they become embrittled and prone to fracture. Under the same loadings, PGSU remains flexible and compliant.
PGSU exhibits stretchable, compressible, and bendable mechanical properties that can be tuned by changing the cross-linking density of the polymer (Figure 3). At a typical cross-linking density and up to 60% w/w API loading, PGSU demonstrates significant flexibility and resilience, where the implant can be folded in half and spring back with full elastic recovery. Formulating PGSU with different cross-linkings can augment the stiffness somewhat, but its flexibility remains intact. At a typical cross-linking, the bend radius of PGSU and API-loaded PGSU remains unchanged across a variety of model drugs and loadings, demonstrating mechanical compliance that is key for patient comfort when implanted subcutaneously.
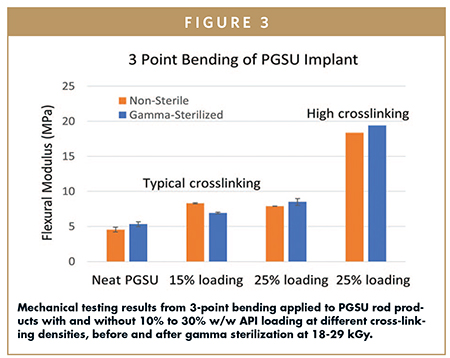
BIOCOMPATIBILITY & PATIENT SAFETY INDICATIONS, INCLUDING RETRIEVABILITY
Implants often suffer from a persistent inflammatory response that leads to increased incidence of fibrosis and fibrous encapsulation, which hinders drug release rates, drug permeation into target tissues, drug distribution within target tissues, patient comfort, patient mobility, implant retrieval, and implant location identification.
Compared to less cross-linked PGSU and even other biodegradable polymers, Secant Group’s range of cross-linked PGSU results in an implantable polymer with reduced inflammatory response, complement activation, cellular attachment, and fibrous encapsulation. This may be due to fewer free functional groups present on the surface that are known to aggravate inflammatory cells, circulating cells, and local cells, and activate complement response. Another reason could be due to slower oxidative degradation, generating fewer free radicals that are known to be pro-inflammatory.4,5 As PGSU surface erodes, the implant material remains biocompatible and non-inflammatory throughout its lifespan, leaving behind minimal changes to the underlying tissue when the implant is 100% degraded.
Unlike other PGSU processes that target lower cross-linking, the reduced cellular attachment to Secant Group’s PGSU creates an implant that has minimal biological interaction with surrounding tissues and more efficient drug release unhindered by cellular adhesion and growth.6
PGSU demonstrates a zero-to-minimal inflammatory cell presence as indicated by the absence of lymphocytes and macrophages, minimal fibroplasia, and no fibrous encapsulation after 12 weeks of implantation (Figure 4). Additionally, PGSU demonstrates zero cytotoxicity, zero acute systemic toxicity, zero irritation, zero subcutaneous implantation side effects, and zero intramuscular implantation side effects, per ISO 10993 and USP Class VI test methods.
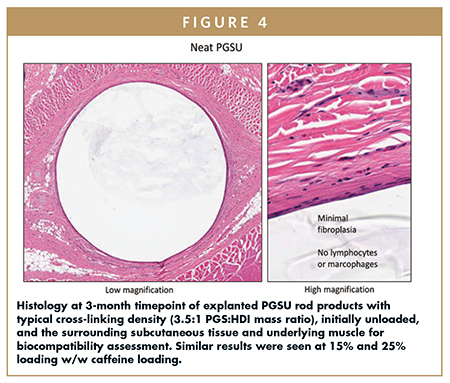
The surface erosion behavior of PGSU allows the implant to remain intact and retrievable for a much longer proportion of the implant lifetime compared to bulk-degrading polymers, which become soft and diffuse quickly. The retrievability afforded by PGSU is important in the instance when a patient has an adverse reaction to the API, needs to receive oral or intravenous therapy that is contraindicated with the drug delivered by the implant, needs to receive therapy that cannot additively stack with the dose delivered by the implant, or otherwise needs emergency removal of the implant for any reason.
SURFACE EROSION PROVIDES LONGER-LASTING, CONTROLLED DRUG RELEASE
As a surface eroder, PGSU demonstrates the ability to maintain near zero-order release kinetics across 10% w/w to 80% w/w loadings throughout implant erosion to 100% degradation, both in vitro and in vivo with good correlation (Figure 5). The release rate of API from PGSU is dictated by the rate of surface erosion. Unlike diffusion-driven non-degradable and bulk-degrading polymers, PGSU does not experience a drug concentration gradient between the internal polymer and external environment. Accordingly, PGSU offers a nearly dose-independent API release rate in which higher drug loading does not significantly impact the rate constant. Cross-linking density instead drives the release rate; PGSU has been shown to maintain a near-constant release rate from 10% w/w to 80% w/w API loadings.
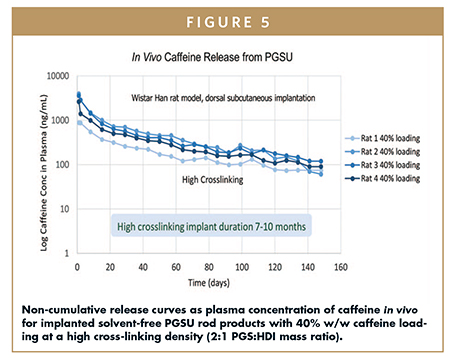
The surface erosion behavior of PGSU allows a therapeutic concentration of a drug to be reached immediately after implantation, in contrast to diffusion-driven non-degradable and bulk-degradable polymers. Specifically, surface erosion reduces the burst release common to hydrophilic APIs and avoids the lag effect common to hydrophobic APIs. Surface erosion allows a rapid onset of degradation at the end of the implant lifetime, reducing the tailing sub-therapeutic concentration that commonly lingers in non-degradable polymers. It also avoids dose dumping that commonly afflicts bulk-degradable polymers. Finally, as mentioned earlier, surface erosion eliminates the need for implant removal, thus improving patient experience.
ACHIEVING SURFACE EROSION AT HIGH DRUG LOADING
PGSU is water impermeable and hydrophobic, as confirmed by water permeability and water vapor transmission rate tests. PGSU is also minimally swelling, and the swelling behavior and sol content change with cross-linking density.
However, when API is loaded into the PGSU matrix, consider how drug particles may impact impermeability of PGSU. Factors such as drug solubility, distribution, particle surface energetics, particle size, loading, wetting by PGS resin, and PGSU cross-linking density all impact water permeation and percolation into and through the PGSU matrix, which in turn affects burst release and release kinetics. Permeability and percolation must be managed to ensure effective controlled drug delivery by surface erosion with minimal diffusion.
Water penetration issues can be mitigated by improving API distribution and reducing agglomerations, which results in well-dispersed API particles that are not initially interconnected so water cannot percolate in. Even with excellent API dispersion, particles can become interconnected above a certain threshold, restricting the maximum drug loading that is still deliverable by surface erosion. This is particularly true for hydrophilic APIs. For hydrophobic APIs, particle distribution and interconnectivity become less crucial due to the drugs’ poor solubility and invulnerability to diffusion.
Water penetration can be further mitigated by increasing the cross-linking density, which results in a smaller polymer mesh size that hinders the permeation of water and APIs through the PGSU matrix. Increasing PGSU cross-linking slows the erosion rate of the thin polymer walls separating API particles, delaying the formation of interconnected ingress channels that emerge during degradation.
As long as the API is well-distributed and loaded below the percolation threshold, water cannot penetrate into the minimally swelling, hydrophobic PGSU matrix. Only the outer circumference of PGSU matrix is accessible by water, and thus surface erosion by hydrolysis occurs inward layer by layer.
SUMMARY
Secant Group’s PGSU outperforms commercial polymers as the only implant that is simultaneously tunable, flexible, surface-eroding, and capable of sustaining release at high-drug loadings for many months. Taken together with the biocompatibility and shelf-life stability of PGSU, pharmaceutical formulators now have improved and expanded polymer options for long-acting delivery using PGSU to achieve unprecedented therapy durations and deliver challenging APIs.
REFERENCES
- Barrett, S., Teller, R., Forster, S. et al., Extended-Duration MK-8591-Eluting Implant as a Candidate for HIV Treatment and Prevention. Antimicrob Agents Chemother. 2018;62(10). doi:10.1128/aac.01058-18.
- Nicholson, C. B., inventor; Secant Group, assignee. Water-Mediated Preparations of Polymeric Materials. US patent 9,359,472. Issued June 7, 2016.
- Reed, S., inventor; Secant Group, assignee. Tunable, Controlled-Release, Urethane-Containing Elastomers and Processes of Forming the Same. US patent application 16/547,175. Filed August 21, 2019.
- Tang, L., Liu, L., Elwing, H.B. Complement Activation and Inflammation Triggered by Model Biomaterial Surfaces. J Biomed Mater Res. 1998;41(2). doi: 10.1002/(sici)1097-4636(199808)41:2<333::aid-jbm19>3.0.co;2-l.
- Conner, E.M., Grisham, M.B. Inflammation, Free Radicals, and Antioxidants. Nutrition. 1996;12(4). doi: 10.1016/s0899-9007(96)00000-8.
- Pereira, M.J.N., Ouyang, B., Sundback, C.A., et al. A Highly Tunable Biocompatible and Multifunctional Biodegradable Elastomer. Advanced Materials. 2012;25(8). doi:10.1002/ adma.201203824.

Stephanie Reed is the Director of Advanced Biomaterials Development at Secant Group, garnering 13-plus years of experience in biomaterial drug delivery for pharmaceuticals and medical devices. Dr. Reed earned her BS in Mechanical Engineering with a Biomedical Engineering minor at Massachusetts Institute of Technology, and her MS and PhD in Biomedical Engineering at the University of California Los Angeles.

Carissa Smoot is a Scientist II in Advanced Biomaterials Development at Secant Group. Since earning her BS in Chemistry from the University of Delaware, she has spent her time at Secant conducting and scaling polymer syntheses, optimizing polymer formulations, and developing coating technologies. She is now an integrated contributor to Secant’s drug delivery platform.

Dennis Shull is an Associate Scientist in Advanced Biomaterials Development at Secant Group. He earned his BS in Biochemistry, Molecular Biology, and Chemistry from Ursinus College, and has 3-plus years of experience characterizing biomaterials and developing analytical methods for drug delivery applications.
Total Page Views: 6724










