Issue:April 2022
PLATFORM TECHNOLOGY - Predictive Medicine, Biomarkers & the Multiple Unmet Needs in Acute Respiratory Distress Syndrome
INTRODUCTION
Precision Medicine is typically used to describe therapeutic strategies to benefit a particular group of patients that are optimized by using genetic, biochemical, or molecular profiling. It has often been simply stated as the “delivery of the right drug to the right patient at the right time.” From a translational research perspective, biomarkers are an important part of the precision medicine approach not only clinical trial design, but also a relevant part of drug utilization upon FDA approval. As an example, HER2+ breast cancer tissues express high levels of a protein called human epidermal growth factor receptor 2 (HER2), which accelerates cancer cell growth and is the underpinning for the observed aggressive behavior of HER2+ breast cancer. Women with breast cancer are tested for this specific subtype as there are HER2+-specific medications that address this aggressive cancer. The use of this genetic assay is a type of biomarker certainly more common in oncology that enhances the likelihood of a response to a specific therapy. This identification of HER positivity in expression frames the notion of “personalized” or “predictive” medicine for highly specific cancer therapies. Unfortunately, the application of personalized or predictive medicine approaches have yet to be adopted as part of the drug development paradigm for active drug utilization and patient stratification for the vexing and often lethal inflammatory condition known as Acute Respiratory Distress Syndrome (ARDS).
ARDS & THE MULTITUDE OF UNMET NEEDS: CRIPPLING GAPS BETWEEN CLINICAL DISCOVERY & UTILITY
Prior to the COVID-19 world-wide pandemic, caused by the species-jumping novel coronavirus SARS-CoV-2, more than 700,00 individuals (at press time) in the US and 2 million cases globally (at press time), annually developed ARDS from trauma, sepsis, bacterial, and viral infections, exhibiting a cumulative mortality of 40%. The root cause of ARDS mortality is unchecked inflammation with exposure to mechanical ventilation, which is nearly universally required in ARDS patients, significantly contributing to the inflammatory burden of ARDS and ARDS mortality, a complication known as ventilator-induced lung injury (VILI). The COVID-19 pandemic has highlighted multiple unmet needs in ARDS. These include the absence of effective FDA-approved pharmacotherapies as neither SARS-CoV-2 vaccines or anti-SARS-CoV-2 drugs address the unchecked inflammation that drives multiorgan dysfunction and ARDS mortality.
Despite recent innovations in translational research and an exponential increase in the discovery of novel biomarkers, precision medicine approaches have yet to be applied to ARDS, and validated ARDS biomarkers remain absent. This critical unmet need in ARDS exposes a critical gap between the fast pace of biomarker discovery and the successful translation to clinical use, and highlights the need of biomarkers to impact a more streamlined drug approval process. The lack of reliable and validated ARDS biomarkers is a contributor to the myriad reasons for the failure of clinical trials in ARDS. ARDS heterogeneity contributes to the requirement for large numbers of enrolled subjects to demonstrate clinically significant benefit. A major value for precision medicine approaches is the reduction in subjects required to demonstrate clinical benefit. Stratification of ARDS patients with reliable biomarkers that are predictive of mortality would optimize participant selection for clinical trial enrollment by focusing on those subjects most likely to benefit from novel clinical interventions. More than 45 promising candidate biomarkers in ARDS have been described in the medical literature; however, to date, no biomarker has been successfully developed as an accepted point of care surrogate marker of disease.
COVID-19 INDUCED ARDS & PRO INFLAMMATORY CYTOKINES
These unmet needs that exist in ARDS are particularly highlighted by the unprecedented numbers of COVID-19-induced ARDS cases, which has strained healthcare systems across the world and exposed the need for biomarkers that would accelerate drug development and successful phenotyping of COVID-19-infected patients at risk for development of ARDS and ARDS mortality. Only 5% of COVID-19-infected patients develop ARDS, require mechanical ventilation, and are at high risk for multiorgan failure and death. Insights into promising stratification-enhancing, biomarker-based strategies in COVID-19 and non-COVID ARDS may enable the design of successful clinical trials of promising therapies. IL-1b, TNF-a, IL-6, IL-1, and IL-18 have been associated with increased mortality from ARDS. However, none of these pro-inflammatory biomarkers have sufficient specificity to serve as a stand-alone prognostic biomarker. Higher plasma levels of IL-6, IL-8, IL-1RA, measured as part of a panel of 6 biomarkers were associated with increased risk of mortality. Unfortunately, no single biomarker has been shown to reliably provide information about the patient’s overall disease outcome, and thus stratify patients for enrollment in clinical trials. However, recent efforts to combine biomarkers demonstrate that prognostic ability can be greatly enhanced. Sadly to date, there have been more failures in clinical trials of therapeutic drugs to treat hospitalized COVID-19-induced ARDS patients.
Several high-profile efforts targeting IL-6 and IL-6 receptor antagonism (Tocilizumab-Roche/ Sanofi, Sarilumab-Regeneron/Sanofi), in COVID ARDS failed to improve COVID-19 ARDS mortality in Phase 2/3 clinical trials of COVID-19 patients with severe disease. With runaway inflammation being an inherent problem associated with COVID-19 -induced ARDS patients, many of the proposed therapeutic drugs have focused on an individual cytokine (IL-6) or pathway that can play an integral role in addressing unchecked inflammation. While a reduction in one individual cytokine (biomarker) can play an important role, all too often it may not be enough to address some of the other elements of unchecked inflammation, and therefore can lead to a failed clinical trial and unmeaningful benefits to patient outcomes.
The absence of successful ARDS clinical trials that target inflammatory pathway components poses a vexing problem that potentially implicates several issues, including: (1) poor target selection, that is, targeting cytokines downstream in the inflammatory cascade, and (2) delayed administration of the anti-inflammatory therapeutic, that is, at a point where the capacity to influence the severity of inflammatory cascade activation is minimal.
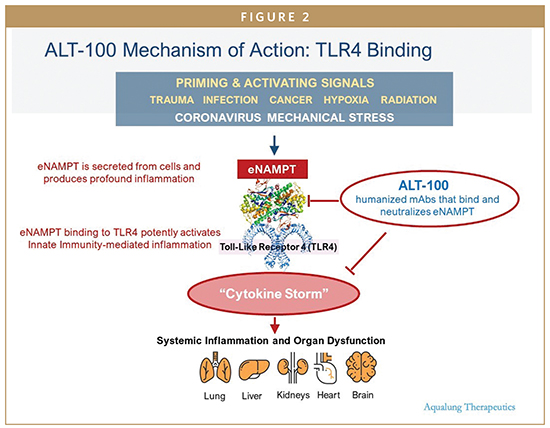
ENTER EXTRACELLULARLY SECRETED NICOTINAMIDE PHOSPHORIBOSYL-TRANSFERASE (ENAMPT) AS A NOVEL COVID-19 ARDS TARGET
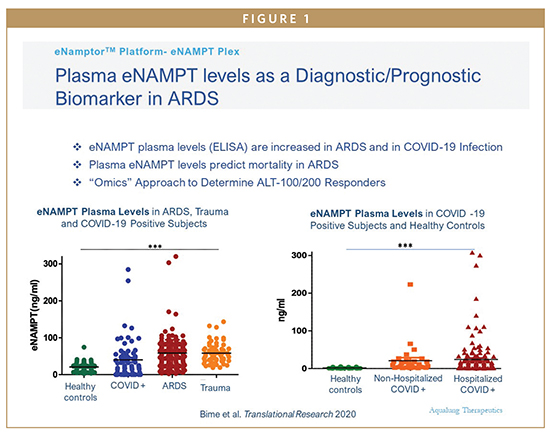
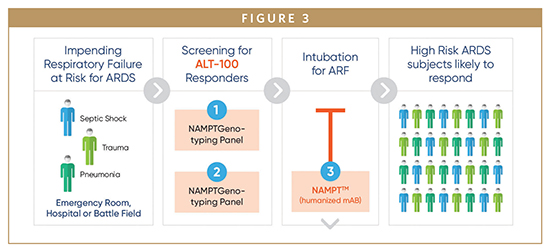
We have previously utilized genomic intensive approaches and cellular and preclinical studies of bacterial pneumonia and excessive mechanical stress/ventilator-induced lung injury (VILI) to identify eNAMPT as a novel damage-associated molecular pattern protein (DAMP), a class of immune-related proteins that serve as sentinels for bacteria or viral infection. Circulating eNAMPT is an essential participant in ARDS/VILI pathobiology and functions as a master activator of evolutionarily conserved inflammatory cascades. eNAMPT is an ARDS biomarker, as high eNAMPT levels at the time of admission to the intensive care unit correlate with disease severity and may predict mortality in patients with sepsis and ARDS. Furthermore, genetic variants in the NAMPT gene, known as single nucleotide polymorphisms or SNPS, are associated with an increased risk of developing sepsis/trauma-induced ARDS/VILI and increased ARDS mortality. eNAMPT, a highly druggable target and a humanized eNAMPT-neutralizing mAb (ALT-100), has been developed that has been proven to be efficacious in preclinical ARDS/ VILI models. Thus, an eNamptorTM precision medicine platform comprised of a plasma biomarker test, a genetic test, and a specific monoclonal antibody, has the potential to serve in clinical trial design that applies precision medicine strategies in ARDS, perhaps ending the drought for effective FDA-approved drugs in ARDS.
SUMMARY
In ARDS, the unmet need is to identify reliable, validated ARDS biomarkers that minimize ARDS heterogeneity and allow for stratification of subject selection for enrollment in clinical trials of tailored therapies. Combined with a more streamlined drug-approval process, biomarker- and genotype-based treatment of specific ARDS endotypes has never been as within reach as it is today.

Dr. Joe “Skip” G.N. Garcia is the Founder & Chief Executive Officer of Aqualung Therapeutics. He is a University of Arizona Endowed Merlin K. DuVal MD Professor of Medicine and an elected member of the National Academy of Medicine. Dr. Garcia is an internationally recognized physician-scientist with over 30 years of research experience in pulmonary disease and has led multi-billion-dollar academic organizations. He is a leading authority on the genetic basis of inflammatory lung disease with emphasis on health disparities, particularly of the underserved minorities. He has over 500 peer-reviewed publications to his credit, has an expansive portfolio of NIH-sponsored research, and continues to direct large federally funded programs. He is a passionate advocate for the training of physician-scientists and is an active supporter of minority medical and science students. Dr. Garcia is internationally recognized for his development of novel therapies for critically ill patients with acute inflammatory lung disease holding over patents.

Stan Miele is President of Aqualung Therapeutics. He is a recognized global executive with success in sales, marketing, and P&L leadership in the pharmaceutical/medical device and biotech industries. He was formally the Chief Commercial Officer at bioLytical Laboratories and Sucampo Pharmaceuticals Inc. He was also President of Sucampo Pharma Americas for 6 years. He was instrumental on some key licensing agreements for Sucampo, inclusive of the agreement with Abbott Japan, and also Takeda Pharmaceuticals (now Shire). He is actively part of the team ensuring proper execution of clinical development, manufacturing, licensing, capital funding, alliances, and ensuring Aqualung meets all critical milestones. He will be helping the company move toward accelerating the pipeline/platform technology and moving the eNamptorTM platform toward commercialization.
Total Page Views: 3508










