Issue:April 2023
PLATFORM TECHNOLOGY - An Alternative Solution for Peptide Drug Formulation
INTRODUCTION
There is a new tool set available to drug formulation scientists that brings solutions to several drug development problems posed by the properties of peptide drug substances. It is the purpose of this review to introduce the technology platform and provide examples of how it has solved difficult formulation problems while adding significant commercial value to the resulting drug products.
PEPTIDE MOLECULES PRESENT CHALLENGES FOR FORMULATORS
Often in the long process of developing a new drug, a great deal of time, money, and effort are spent at the computer and in synthesis labs trying to develop tweaks to a candidate molecule that will improve its solubility, stability, or impart some other property that makes it suitable for a particular delivery route. This is particularly true with peptide molecules. The seminal work of Dr. Bruce Merrifield, in developing a solid-phase sequential synthesis scheme for polypeptide molecules, spawned a revolution in medicinal chemistry and drug development.1 Merrifield’s work and that of those who followed, enabled peptide molecules and analogs to be synthesized quickly, inexpensively, and in sufficient quantity to be evaluated for biological activity. Whether looking at the nervous system, the circulatory system, hormonal systems, the immune system, or virtually any other life process, peptides have been identified that play significant roles, forming the basis for their vast and expanding potential as drug candidates. Peptide-based drugs now generate several billions of dollars in annual pharmaceutical revenues, and looking across industry and academia, one can identify hundreds of new candidates in various stages of development at any given point in time.
A significant limitation of peptide molecules as pharmaceutical agents arises because they are subject to degradation by several mechanisms during isolation, formulation, storage, and handling. Peptides are particularly labile to water and pH, which are also essential to their function. Water-mediated degradation pathways are arguably the most significant set of factors that impact peptide drug formulations. Hydrolysis, aggregation, fibrillation, deamidation, and other side chain reactions are all water and/or pH mediated.2 To preserve function in drug formulations, many if not most, peptide and protein-based drug products are formulated as lyophilized products to keep them intact and away from water until they are rehydrated at the point and time of use. Because of these propensities to degradation, some naturally occurring peptides with significant potential for therapeutic uses have not been able to be developed as drugs. Examples include human amylin, an important pancreatic hormone that works in partnership with insulin to control blood glucose levels. While the insulin peptide became a widely used therapy for diabetes, the natural amylin peptide was not amenable to formulation as a drug because it quickly forms insoluble aggregates and gels.3 Glucagon, a third natural peptide involved in glucose metabolism, has been isolated and used as a drug, but must be quickly formulated and lyophilized to retain solubility. Once lyophilized glucagon is rehydrated, it has just minutes of useful life before it forms aggregates and precipitates. Another example of a potential therapeutic that was not amenable to formulation for drug use is the human calcitonin peptide, which plays an important role in maintaining bone health. When isolated and placed in aqueous solution at concentrations useful for drug delivery, human calcitonin quickly forms fibrils and precipitates.
A NOVEL APPROACH TO SOLVATION & STABILIZATION
The following discusses a novel formulating approach to solving some of the stability and solubility problems that confront formulators when exploring peptide (and some non-peptide) molecules for drug use. Here, we introduce a very simple and elegant idea: Polar aprotic solvents, such as Dimethyl Sulfoxide (DMSO), have now been demonstrated to enable dissolution of problematic peptides, when used in conjunction with appropriate excipients, and stoichiometric amounts of ionic species, while maintaining their chemical and physical integrity and potency in solution for extended periods of time at ambient temperatures. Many drug formulators and development teams have been quick to assume that such solvents are unacceptable for drug use, particularly as injections, but this is in fact not the case. Scientists at Xeris Pharmaceuticals, Inc. have now demonstrated the utility and clinical efficacy of several peptide and non-peptide drugs formulated in aprotic solvent systems. The company has received regulatory approvals to market some of these products in both the US and Europe.
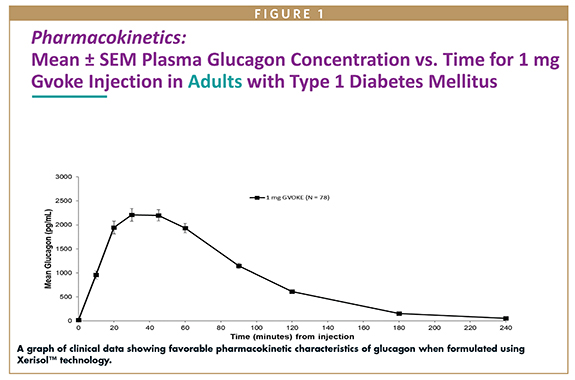
One of the first molecules to be studied by the Xeris team, led by Dr. Steven Prestrelski, was the important metabolic peptide glucagon.4 Glucagon became available as a drug for injection following the advent of insulin therapy, and is provided to insulin users as a rescue drug for acute hypoglycemia. The original formulation was a lyophilized powder that is meant to be reconstituted at the point and time of use, and once reconstituted, the drug must be injected almost immediately or it will aggregate and come out of solution. Glucagon has a very short stability interval once dissolved in water. The Xeris team demonstrated that when formulated in DMSO, along with appropriate levels of other components to enable it to achieve a functional pH when it meets the aqueous microenvironment at the site of injection, glucagon retained stability and pharmacological activity for many months (now 36) as a liquid even when stored at room temperature. This discovery allowed glucagon to be formulated as a ready-to- inject liquid that could be packaged in a pen injector device and used as a “rescue drug” for episodes of hypoglycemia following insulin use. It is noteworthy that throughout their animal studies and human clinical trials, Xeris did not note signs of injection site irritation or pain that were significantly different from the aqueous formulation, and more recent data suggest there are fewer injection site-related complaints with their formulation.5 The Xeris formulation of glucagon is approved by the FDA and is marketed in the US as Gvoke® (glucagon injection) in a single-use rescue pen device and in a prefilled syringe. It is marketed in Europe and the UK as Ogluo® (glucagon injection) in the same packages
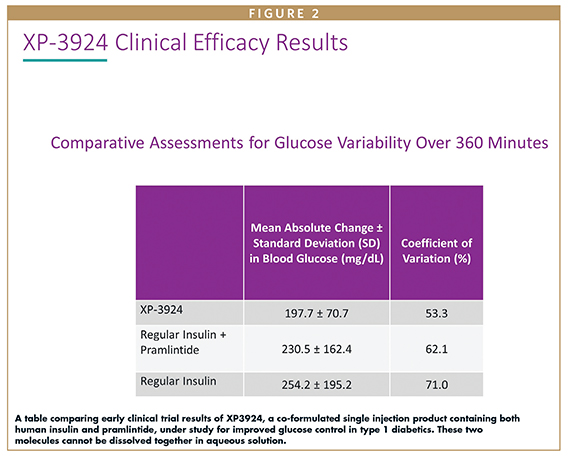
Prestrelski coined the term XeriSol™ to describe this aprotic solvent formulation platform because it incorporates the Greek word Xeris, which means dry, and Sol to indicate solution. The technology platform has since demonstrated another significant benefit in that it allows peptides that require different pH to achieve aqueous solution to be simultaneously dissolved at relatively high concentration. Xeris is now completing Phase 2 clinical trials on a co-formulation of insulin and pramlintide, both of which are deficient in, and which function naturally as partner hormones in healthy glucose metabolism.5 The co-formulated product under study is a liquid subcutaneous injection, which is demonstrating improved glucose control in the study populations. The early clinical data are sufficiently promising, and the company is actively seeking a partner to carry the drug forward into further Phase 2 and Phase 3 studies.
The ability to dissolve multiple peptides in a single solution could yield significant benefits to those seeking to develop “cocktail” drugs, such as multiple peptide antigen vaccines, or other co-formulated peptide combinations that might be imagined for various endocrinological disorders, peptide antibiotic mixtures, etc. It also merits consideration for use by those developing personalized medicines based on peptides, where it may offer a nearly “Plug and Play” formulation approach that yields a commercially viable product sparing the time and expense of complicated lyophilization process development.
SUMMARY
For peptide drug developers seeking to bring benefits to patients expeditiously and perhaps less expensively, this formulation approach would appear to offer significant value. The fact Xeris has assembled an extensive patent estate around the technology platform also creates potential value in that whereas a molecule may not be patentable, such as the case for a natural molecule, a drug created using the patented formulation technology may be protected, and thus enable the developer to enjoy enhanced commercial value for an extended time period. This should spark interest among pharmaceutical companies seeking to extend product life cycles, and also among those seeking to develop and market differentiated biosimilar drugs that are off patent. Xeris is actively seeking licensees for this technology platform, making its potential benefits available to others in the pharmaceutical industry.
Clinically proven success with a non-aqueous solvent system suggests that for a significant problem set confronting medicinal chemists who work in the domain of peptide molecules, the solution may in fact be “the solution” rather than modifying the structure of the molecule, an idea worth considering as they pursue therapeutic goals.
REFERENCES
- Wang et. al. Therapeutic peptides: current applications and future directions Nature, Sig Transduct Target Ther 7, 48 (2022).
- Zapadka, et. al. Factors affecting the physical stability of peptide therapeutics, Royal Society Publishing October, 2017 https://doi.org/10.1098/rsfs.2017.0030.
- US Patent 5686411 Amylin agonist peptides and uses therefor 1997-11-11 Gaeta et al. 514/12.
- Newswanger, et. al. J Diabetes Sci Technol. 2015 Jan; 9(1): 24–33. Published online 2015 Jan 1. doi: 10.1177/1932296814565131.
- Xeris Biopharma Inc. Chicago, IL, United States.

Michael Neely is currently working as a consultant for Xeris Pharmaceuticals, Inc. He is retired from a 42-year career in the Biopharmaceutical industry, with a broad range of experience spanning product development, manufacturing management, marketing, and business development.
Total Page Views: 4239















