Issue:March 2023
PAN-VARIANT INHIBITORS - How Pan-Variant Inhibition Can Outsmart Cancer Treatment Resistance
INTRODUCTION
In targeted oncology, treatment resistance remains a pernicious obstacle to providing patients with therapies that confer lasting benefit. In certain types of cancer, tumors tend to develop mutations that render the cancer unresponsive to currently available targeted therapies, forcing the patient to move to a subsequent line of therapy. Their tumor can often out-mutate every targeted therapy available, one after another, until no effective treatment options remain. At that point, the cancer is free to progress unchecked, and the patient may succumb to their disease.
Fortunately, for many types of cancer in which such serial mutation is common, it is possible to develop targeted therapies that outsmart treatment resistance. The key is to anticipate the finite set of mutations that tend to emerge in a given cancer type, and to develop a single inhibitory agent that binds its target and maintains its anti-cancer activity regardless of the presence of any of those mutations.
To understand how we do that, it’s important to first understand how treatment resistance emerges and why it’s crucial to address in order to successfully treat cancer.
CANCER CELL MUTATIONS CAN LEAD TO TREATMENT RESISTANCE
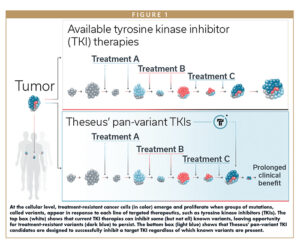
As tumor cells proliferate, they tend to develop mutations more frequently than healthy cells. Some of those mutations confer resistance to certain treatments and are thus termed resistance mutations. These mutations are distinct from activating mutations that drive tumorigenesis in the first place. Resistance mutations often occur at a therapeutic molecule’s binding site on its target protein, such that the molecule can no longer bind to and inhibit its target. The set of resistance mutations a given cancer tends to develop in response to prior therapies is finite, and the sequences and positions of those mutations are often known empirically.
Resistance mutations can often be highly heterogenous — not only between patients, but within individual tumors. As a tumor grows, its dividing cells may partition into distinct clusters of genetically identical cell populations in which each clonal cluster is descended from a different parent cell with a slightly different genome, including perhaps a different collection of resistance mutations.
Additionally, a tumor’s profile of resistance mutations will vary over time as well as space. Tumors tend to accrue new mutations without shedding old ones throughout the course of a patient’s lifetime, and the patient’s population of tumor subclones will often grow and shrink in response to the selective pressures of different targeted therapies. Cells with mutations that confer a selective advantage to the tumor (ie, resistance to the therapy) tend to proliferate more than other cells and occupy larger shares of the tumor’s volume. Cell populations sensitive to the therapy tend to shrink.
So, what does this look like in the clinic? Resistance mutations’ heterogeneity makes them difficult for physicians to both detect and to treat.
Treating resistance mutations after they have emerged is difficult because using a targeted therapy against the most recently emerged mutation is often only temporarily effective until the next mutation occurs.
An often-futile arms race ensues: when a tumor being treated with first-line targeted therapy develops a resistance mutation, a physician moves on to the second-line therapy, to which the tumor may be sensitive, but only until the tumor ultimately develops another mutation that thwarts the second therapy’s effect, and so on through a dwindling arsenal of therapeutic options until none remain.
Without therapies that can remain effective in the face of any mutation the cancer might develop, the patient remains perpetually vulnerable to disease progression.
HOW PAN-VARIANT INHIBITION CAN OUTSMART CANCER TREATMENT RESISTANCE
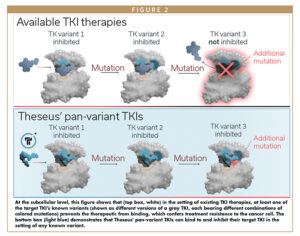
The answer to all these challenges lies in pan-variant inhibition. Pan-variant inhibitors are single therapeutic agents that bind a single target protein to inhibit cancer cell function, and maintain their anti-cancer activity in the face of all major classes of activating and resistance mutations. (“Variant” refers to each of the many possible combinations of resistance mutations that the protein might harbor.)
The goal in targeted oncology is to achieve pan-variant inhibition and, ideally, pan-variant inhibition should be accomplished with a single agent, avoiding the potential complications and toxicities of drug combinations.
Pan-variant inhibitors simultaneously cut off all the tumor’s routes to treatment escape via that target protein, so they can both suppress the emergence of resistance mutations during early lines of therapy, and address those mutations after they have already emerged during later lines of therapy. Thus, they have the potential to provide substantial benefit to patients in both earlier and later lines of therapy relative to current standards of care.
Fortunately, for several cancers prone to treatment resistance, researchers already have a deep understanding of the universe of resistance mutations that emerge in response to current therapies. In many cases, they have also determined the three-dimensional molecular structures of these proteins, so we can visualize the shape of the protein’s surface, identify binding sites for inhibitory molecules, and infer how known mutations might affect the shape of those binding sites.
THESEUS’ APPROACH TO PAN-VARIANT INHIBITION
With this knowledge in hand, we at Theseus apply specialized techniques refined over decades of prior drug development to develop truly pan-variant inhibitors that have the potential to outsmart cancer resistance.
Theseus focuses on inhibiting tyrosine kinases (TKs), a family of enzymes that add phosphate groups to the amino acid tyrosine. This process is fundamental in regulating essential cellular processes, including cell proliferation and cell death, so mutations in tyrosine kinases can contribute to cancer development and progression. In fact, many cancers are driven largely by mutations that accumulate in single tyrosine kinases.
We begin developing a pan-variant tyrosine kinase inhibitor (TKI) candidate by identifying a specific tyrosine kinase that is known to accrue activating and resistance mutations in a given type of cancer.
Next, we use a carefully honed iterative process that optimizes the inhibitory activity of the candidate molecule against all known classes of activating and resistance mutations that particular tyrosine kinase can harbor. Alternating between rounds of structure-guided, in silico molecule design and in vitro activity validation, we refine the molecule’s structure at each iteration until we have optimized pan-variant TKI activity.
We validate the candidate molecule’s inhibitory activity empirically against its target TK using Theseus’ specialized Predictive Resistance Assay (PRA). The cell-based assay, honed over many years by the Theseus team, identifies inhibitors that show pan-variant coverage, ie, a broad range of activity against known activating and resistance mutations in a given cancer. The PRA involves evaluating candidate molecules in a large panel of cell lines in optimized conditions that mimic the human physiological environment. Each cell line harbors a specific resistance mutation of interest. The PRA provides a high-confidence way of assessing whether a given mutation is likely to be inhibited by the candidate molecule in patients.
THESEUS’ PAN-VARIANT INHIBITOR MOLECULES
Since Theseus’ founding in 2017, we have progressed our lead asset into the clinic, where it is being evaluated in gastrointestinal stromal tumors (GIST).
GIST is the most common sarcoma of the gastrointestinal tract with an estimated 4,000 to 6,000 new cases diagnosed in the US each year. About 80% of GIST cases are driven by activating mutations in a tyrosine kinase called KIT, and the disease remains KIT-dependent through successive lines of therapy. Patients typically receive first-line KIT-targeted therapy for GIST, but they often experience disease progression later due to the emergence of other resistance mutations in KIT, which render subsequent lines of targeted therapy significantly less effective. Current standards of care can address some, but not all, of these mutations, leaving an opportunity for GIST patients’ tumors to develop resistance to treatment.
Our candidate in GIST, THE-630, is a small molecule, orally available pan-variant KIT inhibitor, intended for patients whose GIST has become resistant to previous lines of targeted therapy.
Based on our use of the PRA in designing THE-630, the molecule is predicted to achieve pan-inhibition of cancer-causing mutations and resistance mutations known to occur in KIT in the setting of GIST.1 THE-630 will initially be under clinical evaluation for advanced KIT-driven GIST in both the fifth line (for patients who have progressed on prior lines of therapy) and the second line (an earlier stage setting in which there is the potential to interrupt the emergence of resistance mutations).
Our second program is THE-349, a fourth-generation, potent, and selective small molecule designed to inhibit all major classes of epidermal growth factor receptor (EGFR) activating and resistance mutations for the treatment of non-small cell lung cancer (NSCLC).
NSCLC is the most common form of lung cancer, accounting for approximately 85% of the estimated 2.2 million cases of lung cancer diagnosed globally in 2020. Activating mutations in EGFR occur in 10%-15% of Caucasian and up to 50% of Asian NSCLC patients, with up to 90% of those mutations found in exons 19 and 21. In response to treatment, patients’ tumors can develop one or more additional EGFR mutations, causing resistance and rendering current therapies ineffective.
We have demonstrated preclinically that pan-variant inhibition of all major single-, double-, and triple-mutant EGFR variants, including those with the T790M and C797X mutations, with mutant-selectivity and central nervous system (CNS) activity, can be achieved with a single molecule.
Finally, Theseus is also developing a series of next-generation, pan-variant TKI molecules targeting BCR-ABL mutations for the treatment of refractory chronic myeloid leukemia and front-line Philadelphia chromosome-positive acute lymphoblastic leukemia.
REFERENCES
- Rivera, V., Huang, W., Lu, M., Pritchard, J., Dalgarno, D. and Shakespeare, W., 2021. Preclinical characterization of THE-630, a next-generation inhibitor for KIT-mutant gastrointestinal stromal tumors (GIST).

Dr. Tim Clackson is President and Chief Executive Officer of Theseus Pharmaceuticals. He most recently served as President and Chief Technology Officer at Xilio Therapeutics, where he led the development of the company’s technology and product strategy. From 1994 to 2018, he was with ARIAD Pharmaceuticals, where he was President of Research & Development and Chief Scientific Officer. He played a key role in the company’s evolution from early research to a global commercial oncology company, and its subsequent acquisition by Takeda. He led the multi-disciplinary R&D team that internally discovered and developed five clinical-stage product candidates, including ICLUSIG® (ponatinib), approved for patients with treatment-resistant Ph+ leukemias; ALUNBRIG® (brigatinib), approved for ALK+ non-small cell lung cancer (NSCLC); and EXKIVITY® (mobocertinib), approved for EGFR Exon20 insertion+ NSCLC. Prior to ARIAD, he was a post-doctoral research fellow at Genentech. He earned his PhD in Biology from the University of Cambridge, and his BA in Biochemistry from the University of Oxford. He serves as a Director on the boards of Forma Therapeutics, Elevation Oncology, and the Massachusetts Biotechnology Council (MassBio).
Total Page Views: 2377