Issue:October 2020
OVER-ENCAPSULATION CAPSULES - Double-Blind, Zero Bias: Over-Encapsulation — The Right Tool for Blinding Studies?
INTRODUCTION
Double-blinding is the key to pharmaceutical trial robustness and study integrity. While many blinding options are available to drug sponsors and external clinical research partners, over-encapsulation remains a popular choice for its dosing simplicity, trial efficacy, patient accessibility, and cost-effectiveness.
With marketing approval costs for a new drug exceeding $2.55 billion according to Tufts, the need to prove superior efficacy and safety is even more intense compared to an already approved and marketed product.1 Beyond formulation and form, the method for visually blinding trial doses becomes a critical factor in determining efficacy and therapeutic performance. A properly blinded study removes both investigator and patient bias due to awareness of the drug’s source and to suppress potential placebo effects as well.2
ENCAPSULATION INNOVATION
Today’s patient-centric applications are demanding more from capsules than ever before. Fortunately, capsule technology is innovating to keep pace.
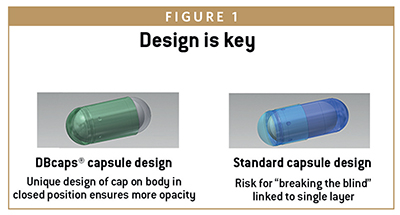
The DBcaps® line of capsules, for example, is specifically engineered for over-encapsulation in double-blinded clinical trial applications. Opaque with an extended cap length and dual locking rings, the tamper-evident design helps accomplish blinding’s two primary functional tasks:
-hide the identity of the trial dose
-prevent people breaking open the capsule to see if they are being treated with a real drug or a placebo
In answer to pharma’s need for innovation and a vegan, clean-label solution, Lonza Capsules and Health Ingredients (CHI) broadened its DBcaps® portfolio to include a Hydroxy Propyl Methyl Cellulose (HPMC) version in addition to the standard gelatin type.
KEY TO ENCAPSULATING SUCCESS
Though simple in practical terms, over encapsulating pharmaceuticals and blinding them for study involve several unique processes that can introduce complexities that may be tough to manage without support.
For example, blinding dose can become complex depending on the dose form. Encapsulating a split or broken tablet is a typical requirement. Similarly, different doses may be combined into the same capsule to meet specified requirements.
The key to successful over-encapsulation is to remember to treat each element of the process with the respect it deserves. From sizing and color to dose security and filling, each requires proper analysis and careful execution. This includes access to an experienced supply team dedicated to the capsule manufacturing process.
When each element of encapsulation is considered and managed carefully, fewer issues with this critical portion of the study are likely to occur at the end of the clinical trial supply chain, such as problems with formulation compatibility, bioavailability, and patient dose compliance.

PRIMARY CONSIDERATIONS FOR OVER-ENCAPSULATION
For clinical conditions in which the bioavailability of the investigational new drug is critical for efficacy, both the investigational and the reference drug should be encapsulated with the same capsule so that appropriate comparisons and conclusions can be drawn. It is important to select the appropriate components that will be needed to support the over-encapsulation of the tablet or capsule unit.
This is essential if one wants to obtain similar in-vitro dissolution profiles. Even when similar overall bioequivalence data (Cmax, AUC) are achieved between non-encapsulated and over-encapsulated tablets, results may not necessarily reflect the drug absorption during the interval after dosing.
SIZE MATTERS
Once the trial dose form has been identified, determining what size capsule shell is needed to properly blind each unit comes next. But there are nuances to this. For example, Lonza CHI has found through experience that efficiencies can arise if the dose being encapsulated does not protrude above the body of the over-encapsulating shell.
If the final dose does not “sit” properly inside the encapsulating shell, backfilling may be required to seat the final dose optimally. When this is required, it may become necessary to over-backfill the capsule, which can raise costs and waste.
FLEXIBILITY REQUIRED
A study design might call for splitting tablets to fit them into the smaller capsule sizes to suit patient group requirements. But splitting doses by breaking tablets physically is not precise and may introduce significant variation. Regulators addressed this by prompting drug developers to address these issues by filling capsules intended to study tablet OSDs with the formulation provided in powdered form to ensure dose accuracy. However, using an equivalent powdered formulation and filling it into capsules prior to blinding can add to program complexity and the demand for more flexible over-encapsulation solutions to fit more dose forms.
Capsule size is an extremely important detail to ensure trial supply dose compliance and blinding performance. To correctly specify the capsule size, one requires data and input from all corners of drug development, trial design, and administration as well as from critical supply, equipment, and fill-finish partners.
COLOR BLIND BY DESIGN
First and foremost, the color must completely hide the enclosed dose form. Ideally, the color and density of the capsule will absolutely blind any “details” of the contents. Anything that signals there is a trial dose inside could impact patient performance. Therefore, capsules for over-encapsulation are generally opaque and usually not the same color or shade of the dose being blinded.
It is vital to select a capsule color to effectively blind the enclosed dose, but it is also important that the color ingredients are accepted by regulators and meet standards wherever the study is being conducted.
Several countries have restrictions on particular colors or the total number of capsules. Compliance issues, including the foregoing, must be researched and understood prior to selecting trial sample colors.
MOTION CONTROL FOR BETTER BLIND CONTROL
Over-encapsulation ensures blind integrity because it completely hides the fact that the control dose is inside. This includes using a backfill excipient so that the trial dose cannot rattle around inside exposing the fact the capsule contains more than just powder or pellets.
If the rattle is not eliminated, the patient can possibly break the blind. Backfill can be avoided in certain cases, but only if a similar rattle between the doses can be maintained.
Lastly, when selecting backfill excipients, it may be convenient to choose one that is present in the dose form being blinded.
DON’T FORGET DISSOLUTION & STABILITY
Probably one of the more critical issues with over-encapsulation selection is its general compatibility with the trial dose, the control dose, and any excipients. Consider the insolubility issues associated with today’s highly potent APIs. Evaluating these factors is more important than ever.
The most commonly used excipients for backfilling are microcrystalline cellulose and lactose monohydrate, used both independently of one another as well as combined in a blend. Research has shown that the combinations of the two can influence dissolution results.
Depending on the grade of the material chosen, a lubricant, usually magnesium stearate (present usually less than 0.5%), is added as part of the backfill formulation. However, not all grades of these two materials require lubrication, and adding the magnesium stearate is usually based on its presence in the formulation of the unit being encapsulated in the first place.
It’s imperative to conduct dissolution profiles and stability work early to verify that the material selected does not interfere with or create any bioavailability issues in the over-encapsulated dosage form.
STORAGE & SHELF-LIFE
Trial dose supply fill and finishers, among others, must also consider shelf-life and similar concerns with encapsulating shells — for some, storage and inventory control are extremely important issues.
Generally, if capsules are stored for more than 2 years, it is good practice to keep fresh supplies. Extended periods of storage in dry conditions can turn capsules brittle and make them prone to manufacturing issues related to the capsule’s degraded physical properties.
Close consultation with suppliers may reveal better-performing gelatin capsule materials or alternatives that deliver both good performance and meet the industry’s increasing quest for cleaner-label ingredients.
MORE OPTIONS, LESS CONFLICTS: GELATIN & HPMC
Developers of therapeutics of all kinds are responding to emerging social and cultural trends. These include increasing consumer demand for products free from any animal proteins and with colors and ingredients derived solely from natural sources.
Gelatin-based capsules offer traditional cost and functional benefits and remain the gold standard for pharmaceuticals and therapeutics of all kinds. The benefits supported by data to prove compatibility across thousands of applications.
However, emerging clean-label requirements are now starting to dictate that products should be free from animal proteins or colourings derived from artificial sources. With potent NMEs under development, challenges deploying APIs in gelatin-based capsules are also contributing to a shift toward the use of HPMC-based capsules. Issues with cross-linking reactions and difficulty containing hygroscopic APIs top the list of these challenges.
For a broad segment of drug delivery strategy, HPMC-based capsules show great potential in becoming the best-practice alternative to gelatin-based formulations, not only because of their provenance, but also for their performance.
SUMMARY
Over-encapsulation remains a widely used, highly effective technique for blinding solid oral dosages in comparative clinical trials. Compatibility is foremost. Dissolution, diffusion, and stability studies are essential elements of selection and therapeutic performance. In addition, patient-centricity is shaping the selection of materials and specifying criteria, leading to the introduction of innovative clean-label products like HPMC-based capsules compatible with vegetarian and vegan lifestyles.
Although HPMC-capsules offer a range of development benefits, traditional hard-gelatin capsules are still the first choice for blinding studies. However, more viable choices are available now than ever before, which increase sponsor flexibility and give them greater freedom in patient trials and fewer compromises regarding product compatibility and format.
REFERENCES
- https://www.scientificamerican.com/article/cost-to-develop-new-pharmaceutical-drug-now-exceeds-2-5b/.
- https://pubmed.ncbi.nlm.nih.gov/26044462/https://www.jforcs.com/7/wpcontent/uploads/2018/06/Unmasking-the-blind-of-over.pdf.

Julien Lamps is Product Manager at Lonza Capsules and Health Ingredients. He graduated from Ecole Nationale Supérieure de Chimie de Lille with an Engineering degree in Chemistry in 2004. He joined Capsugel as a Quality Assurance Engineer in the Colmar plant in 2011, and in this role, he worked at the interphase of operations and customers within the well-known Capsugel® Quality Mindset. During this time, he specialized in coordinating new product introductions to develop innovative offers around modified-release profiles and inhalation products.
Total Page Views: 11283










