Issue:September 2023
MEDICAL DEVICE TESTING - Breathing Component Biocompatibility: The Practical Application of ISO 18562
INTRODUCTION
The Medical Devices Industry has grown and is expanding daily. With this in mind, manufacturers must prove the safety and effectiveness of the products before being used in humans. All over the world, US, EU, and Asia, authorities are reviewing the data considering similar bullet points.
The world has faced the COVID19 crisis, and during these times, many medical device manufacturers have designed breathing devices to save lives. The safety of these ventilators had to be proven and the risk versus benefit measured.
Respiratory devices are classified as indirect contact medical devices as per ISO ISO10993: Biological Evaluation of Medical Devices and are being assessed following ISO18562: Biocompatibility evaluation of breathing gas pathway in healthcare applications standards.
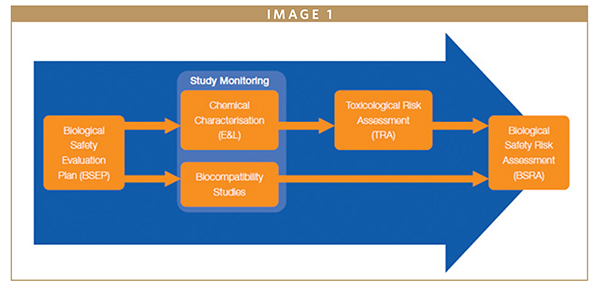
Even if breathing medical devices are assessed by a different standard, the process to submission is similar to direct medical devices. The first step is assessing the risk, and for this, information gathering is mandatory for the Biological Evaluation Plan (BEP). At this stage, the next steps are defined. If the information is incomplete or the risk is not minimized, further testing is required.
For contact medical devices, extractables and leachables studies, degradation studies (when the potential for degradation exists), chemical characterization, and often animal testing, are required as per ISO10993: Biological Evaluation of Medical Devices.
For indirect contact medical devices, specifically breathing devices, a similar approach is required. The main difference is the assessment of the potential extractables and leachables is performed using gas to simulate real-life scenarios. The main focus is to gather data about any materials that could be released in the gas pathway and inhaled by the user. While in direct contact medical devices in which the chemical data is generated using extraction solvents, temperature, and different time conditions, breathing medical devices are tested by simulating the worst-case scenarios, and instead of using extraction solvents, the “extractables and leachables” released into the gas pathway are analyzed, and the extraction vehicle in this study is the air.
THERE ARE 4 PARTS OF THE STANDARD
ISO18562-1: Biocompatibility Evaluation of Breathing Gas Pathways in Healthcare Applications – Evaluation & Testing Within a Risk-Management Process
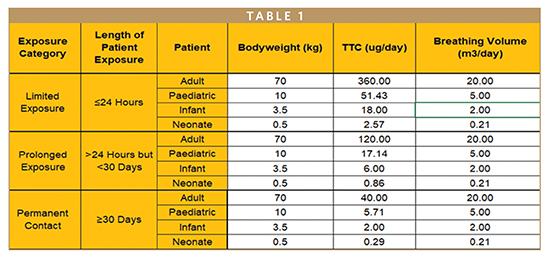
This document presents the key steps that need consideration when assessing breathing devices. The most important is the information gathering process and the Biological Evaluation Plan before any testing is performed. The standard guides on the different types of patient groups, breathing volumes, body weight, and Threshold of Toxicological Concerns.
ISO18562-2: Biocompatibility Evaluation of Breathing Gas Pathways in Healthcare Applications – Tests for Emissions of Particulate Matter
The standard is presenting different ways of simulating the worst-case scenario for any particulate matter to be released from the medical device and that can land on the patient’s lungs. For this test, the highest flow rate is considered as the worst-case scenario as more particulates can be released when the device is working harder to provide more air. Only particulates with a diameter more than 0.2 μm are of interest for this study. There is a maximum allowable mass limit of 12 μg/m3 of accumulated particulate mass, without differentiating the size.
When the size of the particulates is differentiated, the limit for the particulates with a size between 0.2 μm and 2.5 μm has a maximum limit of 12 μg/m3, while the mass of particulates with a size between 2.5 μm and 10 μm cannot exceed 150 μg/m3. The evaluation must consider the expected service life, any expected or processing or reprocessing, and patient contact.
ISO18562-3: Biocompatibility Evaluation of Breathing Gas Pathways in Healthcare Applications – Tests for Emissions of Volatile Organic Compounds (VOCs)
In this part of the standard, the focus is on any volatile organic compounds that can be released by the breathing device during use. As some volatile organic compounds (VOCs) can become gas at room temperature, and to simulate the worst-case scenario, testing must be performed at the highest clinically relevant temperature. This accelerates the volatilization of any potential harmful materials. The flow of the testing is also one crucial parameter to consider, and the lowest clinically relevant flow is considered as the most appropriate as it slowly allows the volatiles to be released and be absorbed.
As per ISO18562-1, different patient groups breathe different volumes of air per day and have different body weights. The permitted concentration of the volatiles is adjusted considering these details. The standard states that any materials below 2 μg/m3 are not to be reported.
ISO18562-4: Biocompatibility Evaluation of Breathing Gas Pathways in Healthcare Applications – Tests for Leachables in Condensate
This part of the standard only applies when there is potential for condensation to form during the clinical use of the medical device. The rationale is that the condensation could lead to materials leaching from the device’s pathway and contacting the patient. The standard presents the testing requirements, and testing involves a polar (water) extraction performed at body temperature (37°C) of the gas pathway components. If possible, the device should be run as in real-life and the condensate to be collected and tested. When this is not possible, an extraction of the relevant parts is performed. The extract is followed by a screening for semi-volatile organic compounds and metals. As part of the study, cytotoxicity and sensitization must be assessed as there are not known in-vitro adequate methods.
Same as ISO10993, ISO18562 classifies medical devices based on their duration of use in direct or indirect contact with the user:
- Short-term exposure or limited exposure: Medical devices whose sum of single, multiple, or repeated duration of use does not exceed 24 hours.
- Prolonged exposure: Products whose cumulative sum of single, multiple, or repeated contact time is likely to exceed 24 hours but not likely to exceed 30 days.
- Long-term exposure devices or permanent contact: Cumulative sum of single, multiple, or repeated contact time exceeds 30 days.
Knowing the duration of use of the medical device is very important as this dictates the testing periods, mainly for VOCs in which ISO18562-3 splits the duration of the testing based on the duration of use.
SUMMARY
At the end of the testing period, all the data is captured and must be assessed in the Toxicological Risk Assessment in which all materials are evaluated for any toxic effects. If toxicity data is not available, TTCs presented in ISO18562-1 are used.
When the end points of the Toxicological Risk assessments are addressed and the device is deemed as having an acceptable toxicological risk from clinical use, the Biological Evaluation Report can be put in place, reviewing that all the recommendations made in the Biological Evaluation Plan have been addressed and the device is safe to be used.
The Biological Evaluation Report is the last step in the process and all the data is reviewed and concluded if the results are satisfactory. In conclusion, even if breathing medical devices are not captured by ISO10993 standards, the approach of proving the safety is identical.

Luminita Moraru is the Analytical Chemistry Manager at Medical Engineering Technologies Ltd. with 7 years of experience in Medical Device Testing. She is an individual expert committee member of ISO10993: CH/194 Biological Evaluation of Medical Devices and ISO18562: CH/121/09 Lung Ventilators & Related Equipment having insight knowledge in the applications of those on medical devices to meet the requirements, ensuring the data is generated in appropriate form to be risk assessed in Toxicological Risk Assessments. She earned her Master’s in Chemistry at the University of Bucharest and is a Member of Royal Society of Chemistry.
Total Page Views: 3750










