Issue:September 2022
LIVE BIOTHERAPEUTIC PRODUCTS - Not All Microbiome Approaches Are Created Equal
INTRODUCTION
The microbiome is the complex ecosystem of microorganisms that live on and in the human body, including bacteria, fungi, archaea, and viruses. Weighing around 2 kg and estimated to contain around 100 trillion bacterial cells representing thousands of species, bacteria in the human gut microbiome outnumber human cells in the body.
All this material is home to vast genetic diversity and functional output. The estimated 2 million bacterial genes in the average human body dwarfs the approximately 20,000 protein-coding human genes. This genetic abundance and breadth, but more importantly its outputs, represent a vast and rich pool of functionality, already optimized by evolution to impact human pathways, and to be leveraged for therapeutic effect.
THE MICROBIOME: DIFFERING APPROACHES TO A NEW MODALITY
The microbiome is a rapidly maturing area of research, and multiple companies are pursuing various strategies to exploit the microbiome for therapeutic benefit. While these strategies are rooted in the same source, they diverge significantly in terms of scientific thesis, proposed mode of action, and development complexity.
Live Biotherapeutic Products (LBPs) are a recognized class of drug, defined by the US FDA as “a biological product that contains live organisms, such as bacteria, that is applicable to the prevention, treatment, or cure of a disease or condition of human beings, and is not a vaccine.” This is an important distinction from both fecal material transplant procedure and small molecule or biologic drugs also derived from the microbiome.
Within the broad umbrella of “microbiome therapeutics,” distinct schools of thought have emerged regarding how best to tap into the microbiome for therapeutic applications. Different rationale often underlies the different approaches taken in microbiome research and has implications for drug development.
Recent years have seen rapid maturation of the microbiome therapeutic landscape, with numerous drug candidates representing a range of approaches in clinical studies for a wide variety of diseases. This has been assisted by an evolution in the way companies are thinking about the microbiome and LBPs as pharmacological agents. A closer examination of the various approaches helps define the microbiome space.
FECAL MICROBIOTA FOR TRANSPLANTATION (FMT)
FMT involves the transfer of a sample of the microbiota from a donor into the gastrointestinal (GI) tract of a recipient, to repopulate the recipient’s “dysbiotic” microbiome. This typically involves antibiotic pretreatment of the recipient to remove the resident microbiota. FMT is the most basic form of microbiome therapy, in which the entire microbiome is transferred wholesale in an attempt to recreate a specific microbiome signature that is associated with a healthy state. Screening of the fecal material is required to identify pathogens, but otherwise the microbial composition of the material is not actively modified in any significant way.
FMT is considered by the FDA to be a procedure and not a drug product. It has proven successful for preventing recurrence of GI infections such as Clostridium difficile (C. diff). FMTs are also now being tested in clinical studies for a wide range of conditions from cancer to autism; however, such studies are often open-label and poorly controlled and therefore unlikely to provide conclusive evidence.
FMT PRODUCTS & BACTERIAL CONSORTIA
FMT “products” are, however, also in development. These are often referred to as “full spectrum microbiota” products and are considered a type of LBP. Several such products have been successful in late-stage trials for the prevention of recurrence of C. diff infection after standard antibiotic treatment for the initial infection.
A similar approach is to use a cocktail or “consortium” of multiple bacterial strains as the drug product. Such consortia products may be composed of hundreds of strains, by reducing from a full-spectrum donor fecal sample, or as little as three strains fermented separately from cell banks before being combined together in a capsule.
FMT products by definition, and many consortia products currently in development, use donor-derived material. This may prove to have important practical limitations, such as variability in the product due to significant inter-individual variability even within healthy donors, reliability of supply, commercial scalability, and risks of infections caused by the transfer of pathogens from donor to recipient requiring rigorous screening and testing.
Some consortium products are manufactured through the fermentation of each constituent strain separately before being combined into a single drug product. Manufacturing complexity thus increases significantly with the complexity of the product. Complex consortia products may also face potential regulatory issues due to agency requirements that the contribution of each component of a drug product be accurately detailed and its inclusion justified.
It may be difficult to tease apart the relative contributions and activity of all strains within a consortium, particularly FMT-like complex donor-derived consortia. The composition of consortia products has typically been based on observations of correlations between the relative abundance of certain bacteria, for example in healthy individuals vs patients with a given disease, rather than known functions of particular strains. As the field has progressed, developers of bacterial consortium products are beginning to consider function more closely, but this still tends to be driven by reverse engineering a mechanism of action based on observations in human studies, rather than direct investigation of specific strains and their impact on host biology.
In developing consortium products, a balance must be struck between, on the one hand, utilizing high-volume dosage forms to ensure a significant number of each strain is administered to the patient (which could adversely affect patient compliance and acceptance of the product, as well as practical considerations of commercial viability), and on the other hand, utilizing more conventional dosage forms (but risking that an ineffective amount of one or more strains will be delivered).

SINGLE STRAIN LIVE BIOTHERAPEUTICS
The history of medicine is typically characterized by refinement from complex beginnings to functional singularity. From plant extracts to small molecules, from smallpox scabs to mRNA COVID-19 vaccines, from whole plasma to recombinantly produced antibodies, and now from FMT to Live Biotherapeutics – drug development has evolved toward more precise approaches with the goal of fundamentally altering mechanisms of disease.
Functionality in complex organisms exists at different levels – organs, tissues, cells, and molecules. Cells represent the simplest unit of independent biological functionality. Likewise, individual strains of bacteria are the simplest unit of contained functionality within the microbiome. Cells-as-therapies have demonstrated the ability to deliver therapeutically relevant effects “in context.” Harnessing and controlling the effects can lead to remarkable results (eg, CAR-T, stem cell therapy, and single strain Live Biotherapeutics).
Selection of a single strain Live Biotherapeutic begins by looking for specific activity against human targets. A panel of candidate strains (originally isolated from healthy human gut microbiome samples) is screened for a particular desired activity or attribute. This may be genetic, metabolic, or through specific functional assays, and such assays may be focused on molecular (activity against a known receptor target), cellular (such as a particular immune cell type or signaling pathway), or phenotypic activity. This process is analogous to small molecule drug discovery.
Focus then turns to understanding how these individual strains act on host biology to exert a therapeutic effect through detailed characterization of mechanism of action and their interaction with human cells through specific pathways. This rational, target- or functionally driven approach has facilitated the expansion of LBPs beyond GI disease, targeting immune-related disease, cancer, and even the central nervous system (CNS).
An additional but often overlooked advantage of single strain LBPs is they can be delivered in a patient-friendly oral dosing regimen. This is in contrast to consortia bacterial products that may involve a high pill burden to deliver a therapeutically relevant dose of each strain. Additionally, because single strain LBPs operate through a well-defined mechanism of action, like a small molecule or biologic, and their activity should be independent of the background microbiota, no pretreatment with antibiotics is required to clear the resident microbiome to make room for colonization.
ENGINEERED BACTERIA
Another quite distinct approach that is typically included within the umbrella of “microbiome therapeutics” because of its use of bacteria as a “delivery chassis” is to engineer bacteria to express heterologous genes for therapeutic activity. A chassis strain is engineered with new, non-native functionality, such as the expression of a therapeutic protein, or heterologous metabolic activity. This approach typically uses well-studied model organisms as the chassis or vector, which have established genetic engineering tools and protocols.
Because these types of engineered bacterial drug products are not naturally occurring organisms, this synthetic biology approach potentially faces a higher safety risk and regulatory hurdle than using wild-type LBPs originally isolated from healthy humans. The FDA’s 2016 guidelines include a sub-categorization for “recombinant LBPs,” which “raise additional considerations and thus would require additional information to be submitted in an IND.”
NON-LIVE BIOTHERAPEUTIC MICROBIOME THERAPIES: BACTERIAL-DERIVED PRODUCTS
Taking the reductionist approach one step further, some companies are looking to bacteria as a source of new drugs, but not as the drugs themselves. This approach centers around identifying and isolating a particular component or molecule produced by a bacterium and developing this molecule itself as the drug product. While this approach does involve bacteria and the microbiome, it is more an expansion of an age-old approach in pharmaceuticals of developing drugs derived from natural sources – think antibiotics first identified from products of other bacteria.
Novel approaches include utilizing biomaterials produced by bacteria, such as extracellular vesicles and small molecule metabolites. One example being pursued is the idea of “molecular mimicry,” looking for bacterial proteins that share structural similarities with human proteins or antigens. For example, in cancer indications, bacterial proteins that share similarities with proteins overexpressed by certain cancers have been identified that could trigger an immune response against those antigens, in a therapeutic approach reminiscent of cancer vaccines.
This approach applies a traditional small molecule or biologic development approach to a new source of compounds, the microbiome. Establishing mechanism of action for an isolated molecule may be easier, and more familiar, than for a whole cell or a complex mixture. Similarly, manufacturing of a biologic or small molecule, regardless of its original source of inspiration, fits more comfortably with established traditional CMC infrastructure and processes.
While this approach may reduce some of the uncertainties around a new modality and use of live organisms, it misses out on some of the novel advantages. Recombinantly or synthetically manufacturing a single molecule and delivering it in isolation negates any benefits from “delivery in context” offered by Live Biotherapeutics. This may include physical localization, such as a bacterium’s ability to penetrate the mucus layer and access human epithelial and immune cells, as well as the context of other co-signaling molecules also being produced by a living, metabolically active bacterium, which may be critical to potentiating or augmenting the activity of the molecule in question.
MICROBIOME-TARGETED DRUGS
Lastly, other companies are approaching the microbiome as a novel target, rather than a source of novel drugs themselves. This typically falls into two categories – subtractive and additive. Subtractive microbiome-targeting therapies are broadly aligned with the historic perception of bacteria as disease-causing pathogens, with the goal of removing or inhibiting specific bacteria, groups of bacteria, their products or activity believed to have a role in the cause or progression of disease. This is typically achieved through small molecules or bacteriophage to specifically kill or inhibit disease-associated bacteria, or molecules designed to sequester, metabolize, or otherwise remove bacterial products also associated with disease.
Additive microbiome-targeting therapeutics are drug products that do not comprise Live Biotherapeutics but are intended to act on the resident microbiome to modulate either its composition or metabolic output. Typically, such products are prebiotics – oligosaccharides that are preferentially used as energy sources by certain bacteria to increase their relative abundance within the microbiome. Similarly, prebiotics may be used to modulate the metabolic output of the microbiome more so than its relative composition. A potential issue for compliance and ultimately the commercial success of medical prebiotics is the large doses required to achieve meaningful modulation of the microbiome. Such prebiotics currently in mid-stage clinical phase are administered in doses measuring tens of grams rather than milligrams.
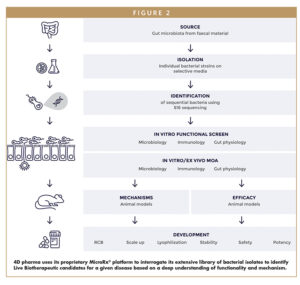
4D PHARMA’S DRUG DISCOVERY MICRORX® PLATFORM
Rather than try to define a “healthy” microbiome, 4D pharma set out to apply the scientific principles and rigor of drug development to this exciting new field, investigating the mechanisms of specific strains of bacteria and their interactions with host biology, and exploiting this in-built therapeutic activity. Based on over 2 decades of world-leading research into the role of the microbiome and its influence on our immune system, 4D pharma has built a proprietary platform – known as MicroRx® – to rapidly select those bacteria that have a therapeutic effect in specific diseases. This sector-leading platform has allowed 4D pharma to uncover the potential of using single strains of bacteria to address a wide array of medical challenges that are not just gut-related but systemic, from GI to cancer to neurodegenerative disorders.
MicroRx is able to rapidly interrogate 4D pharma’s proprietary library of bacterial isolates for specific therapeutic functionality. We understand the microbiome is a complex system often perceived as a “black box,” and therefore the need to demonstrate what the bacteria are doing and how they impact disease biology are critically important for effective drug development. This reflects the natural progression throughout the emergence of any new modality – refining complex mixtures to simpler and more precise therapeutic units. Applying this concept to the microbiome has always been 4D pharma’s focus, and is now increasingly reflected across the microbiome therapeutics space as drug developers move away from “full-spectrum” microbiota products typically based on observational correlations toward more precise, targeted microbiome-derived therapeutics based on mechanisms of action.
PROGRESSION OF LIVE BIOTHERAPEUTICS & CONSIDERATIONS FOR THE FUTURE
In comparison with other therapeutic classes, such as antibodies or gene therapy, the progress that has been made with Live Biotherapeutics to date has been rapid. For the field to maintain this rate of progress and to establish LBPs as a mainstay in the treatment of patients across a variety of diseases, a number of key questions need to be addressed. Compelling clinical data is beginning to emerge in gastrointestinal disease and oncology, but to realize the full potential of microbiome therapeutics, this needs to be continued in multiple settings and larger clinical studies. And as LBPs progress through clinical development toward approval, manufacturing is increasingly recognized as a critical factor to the realization of this new class of drug.
Based on the significant progress that has been made by 4D pharma and others in only the past 10 years, it is realistic to expect that LBPs could soon become an important part of clinicians’ armory for the treatment of many different diseases. The field is tantalizingly close to achieving those all-important first product approvals. It is exciting to be part of the establishment of a new class of safe and effective medicines expected to bring lasting benefit to millions of patients in need and to gain widespread acceptance in the clinical community.

Duncan Peyton has a proven track record in identifying, investing in, and growing businesses within the pharmaceutical sector. He was the founder of Aquarius Equity, a specialist investor in businesses within the life sciences sector, which provided investors with access to innovative, high growth potential companies that delivered significant capital growth. He started his career in a bio-science start-up business, which ultimately went on to list on the London Stock Exchange, subsequently qualified as a corporate finance lawyer with Addleshaw Goddard, then Addleshaw Booth & Co, and later joined 3i plc as an investment manager. He founded Aquarius in 2005, which made founding investments into Nanoco Technologies Limited, Auralis Limited (subsequently sold to ViroPharma), Tissue Regenix Group plc, Brabant Pharma (subsequently sold to Zogenix, Inc) and C4X Discovery plc. He is a Co-founder of 4D pharma plc and has served as Chief Executive Officer since 2014.
Total Page Views: 4778