Issue:April 2023
LIPID-BASED EXCIPIENTS - Misconceptions About Lipid-Based Drug Delivery
INTRODUCTION
For decades, lipid-based drug delivery systems (LBDDS) have been studied, developed, characterized for improving uptake of poorly soluble compounds, found to be safe and effective in delivering a multitude of compounds of diverse chemistries, and present in pharmaceuticals across the globe, including dozens of FDA-approved products.1 Yet, the viability of LBDDS as a platform for modern drug development is still a concern to some due to lingering misconceptions about their functionality, stability, handling, and toxicology. Many of these concerns may be easily addressed by broadening our understanding of oleochemicals’ strengths and sensitivities.
The following aims to provide formulators confidence in using LBDDS as part of formulation development programs, by demonstrating their benefits and key functional mechanisms when used and addressing commonly misrepresented, misinterpreted, and misunderstood LBDDS topics.
UNDERSTANDING MECHANISMS OF ORAL BIOAVAILABILITY ENHANCEMENT FOR LBDDS
Among the mechanisms attributed to lipid-based formulations for enhancing oral bioavailability is natural lipid metabolism in the gut. Known as lipolysis, this metabolic process initiates on contact with stomach fluid thereby releasing gastric lipase. Subsequently, pancreatic lipase and bile salts further break down lipids into micellar structures that are absorbed by enterocytes that line the intestinal tract. Pharmaceutical actives are then solubilized within these lipid formulations and absorbed through the gut in a bioavailable (solubilized) state. This process can be optimized by using digestible surfactants/co-surfactants and oils to create self-emulsifying drug delivery systems (SEDDS) that form fine dispersions on contact with stomach fluid, thereby helping to maintain drug solubility within the emulsion particle.
There are other key mechanisms for enhancing oral bioavailability of actives associated with lipid-based formulations. These include targeting lymphatic absorption to bypass liver metabolism as well as reversable modulation of tight junctions to enhance permeation through the gut lining.
Starting with lymphatic absorption, long chain fatty acids (LCFAs) that are absorbed into the enterocytes can be selectively transported via the lymphatic system. Drugs solubilized within the lipid particles will then avoid first-pass metabolism and will eventually be diverted into the blood stream via lymphatic enterocytes.2 Lipid-based formulations containing esters of LCFAs can also be used to target lymphatic absorption of lipophilic drugs having Log P >5 and solubilities >50 mg/mL in the LCFA based oil as demonstrated in several studies.3
For low-permeability actives including peptides, the reversible opening of tight junctions may assist in the peptide absorption, and with certain lipid-based chemistries, promote the increased uptake of model compounds via the same mechanism. For example, increasing the concentration of materials like Labrasol® (caprylocaproyl polyoxylglycerides) and Labrafac® MC60 (glyceryl mono and dicaprylocaprate) has shown to induce transient opening of intestinal tight junctions to allow the transport of insulin and FITC-Dextran across the epithelial barrier.4,5 Other lipids with medium chain fatty acid chemistries like Capryol 90 (propylene glycol monocaprylate), can enhance the uptake of orally administered model drugs as well.6
OUTDATED CONCEPTS OF INTESTINAL EFFLUX INHIBITION
Given the variety of uptake-enhancement mechanisms inherent in lipid chemistry, LBDDS have historically been evaluated as possible inhibitors of active transport. Previous studies and their conclusions based on antiquated analytical methods that P-gp inhibition occurred with model drugs formulated with permeation enhancers like Labrasol (caprylocaproyl polyoxylglycerides), have recently been shown to be inapplicable as they were based on models no longer considered to be representative of such systems.7
A more recent study suggested that conclusions claiming LBDDS are possible P-gp inhibitors may have misinterpreted other lipid excipient-induced penetration-enhancement mechanisms with P-gp inhibition.8 This study compared the gold standard model from older studies with a more modern version that considered additional parameters, highlighting irregularities between the two. With so many possible mechanisms of bioavailability enhancement intrinsic to lipid-based formulations: participation in the lipolysis process, avoidance of liver metabolism, targeting lymphatic uptake, and reversible modulation of tight junctions, it is difficult to attribute permeation enhancement just to efflux inhibition.
LIPIDS VS. POLYMERS: DIFFERENT CHEMISTRIES, COMPOSITIONS, FORMULATION PROCESSES
While some lipids, specifically those of the chemically modified variety, have overlapping applications with polymeric materials, it is important to understand these chemical families are indeed different. One key distinction is lipids consist of fatty acids, naturally occurring (organic) moieties abundant in plants and seeds, while polymers are composed of chains of repeated units generally derived from synthetic sources. Simple lipids (eg, glycerides) are composed of alkyl heads esterified to fatty acid tails and have well-defined molecular weights, ranging from 8 to 24 pairs of -CH2. Lipid-based materials also generally have lower melting ranges than high molecular weight polymers.
Physico-chemical properties of polymers, on the other hand, are quantified by their molecular weights and highly dependent on the number of repeated units. For example, PEG-1000 and PEG-4000 are both composed of 1000 and 4000 repeat units of ethylene glycol and have different properties due to their vastly different molecular weights.
Also, because lipids are derived from natural and botanical sources, they can range in homogeneity depending on their raw materials (ie, whole natural oil vs. fractionated oil vs. purified fatty acids) and manufacture process design. For example, Labrafil M 1944 CS conforms to the USP monograph for oleoyl polyoxylglycerides and is derived via alcoholysis of apricot kernel oil with PEG to form PEG esters and glycerides of mostly oleic acid, and controlled quantities of stearic, palmitic, and linoleic acids. This is compared to Capryol 90, which conforms to the USP monograph for propylene glycol monocaprylate that is derived via direct esterification of propylene glycol and caprylic acid and distilled to contain >90% monoesters of the raw materials therein. Even though lipid materials have diverse components, this does not make their composition or specifications less consistent than polymers. Like polymeric materials, lipid systems can also have a range of purity depending on manufacturing process and extent of purification (eg, distillation). Lipid-based materials like all pharmaceutical-grade materials in the US must still conform to strict USP/NF monographs.
Lipids’ melt properties and diverse chemistries allow them to be easily incorporated into processing techniques that traditionally use polymer-based systems. Due to the lower melting point ranges of lipid excipients, generally <75°C, they do not require diluting or dispersing in solvents in hot melt processes (eg, melt granulation). They can instead be melted and applied directly onto a substrate without the need for solvents. This means an evaporation step is not required to achieve diverse functionalities like modified release, taste masking, and powder lubrication.9
As another example, lipid excipients can be incorporated into hot-melt extrusion processes but do not undergo a glass
transition like polymer-based materials. Due to lower melting point ranges, lipid-based HME processes do not require the addition of plasticizers because the low viscosities of the melted lipids reduces the need for material shear and leads to reduced energy while maintaining a high throughput.10 Lastly, lipid-based emulsions can undergo lyophilization to form semi-solids that will disperse on contact with aqueous media.11
THE IMPORTANCE OF DRUG SOLUBILITY IN LIPID VEHICLES
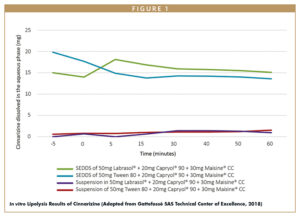
There are several potential ways in which lipid-based chemistries can be harnessed to enhance bioavailability of orally dosed compounds. API solubility in lipids, for example, can be used as an indicator of a formulation’s final performance because compounds solubilized in the lipid vehicle can fully participate in the lipolysis process. In an internal study carried out by the Gattefossé Technical Center of Excellence, Cinnarizine solubilized in SEDDS maintained better solubility in biorelevant gastrointestinal media than Cinnarizine in a suspended state (Figure 1). In other words, a larger percentage of Cinnarizine was in a state that can be absorbed by the body when pre-dissolved in a SEDDS than when dosed suspended in the same vehicles.12 This is a fundamental rationale behind using lipids for oral bioavailability enhancement. It is for this reason that it is highly recommended to complete a solubility screening of actives in an array of lipid vehicles during early stages of development.
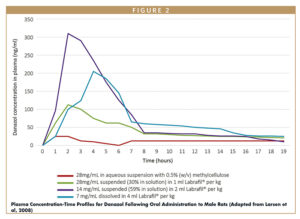
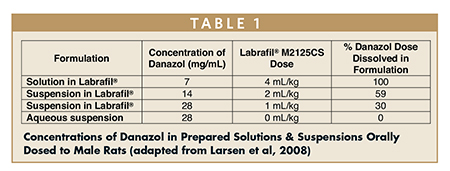
In another example, Danazol (a well-characterized low-water solubility model compound) was orally administered to rats in both a traditional aqueous suspension and in Labrafil M 2125 (Linoleoyl polyoxyl-6 glycerides NF) based solutions and suspensions. Table 1 contains concentrations of Danazol and percentages dissolved in various prepared formulations.
The plasma concentration–time profiles in Figure 2 show a 4-mL/kg solution of Labrafil increased Danazol oral bioavailability nine-fold compared to an aqueous suspension. Note that even lipid-based suspensions of Danazol showed high uptake. It is important to highlight the lipid-based suspensions contained considerable percentages of drug in solution creating a combination of lipid-based solution/suspension compared to the aqueous suspension control.13
While the Labrafil solution showed a similar increase in bioavailability compared to the Labrafil suspension with partial solubilization, increases in bioavailability tend to be dependent on the chemistry of the active compound as well as the dosing methodology based on Gattefossé’s experience with similar studies.
EFFECT OF EMULSION PARTICLE SIZE ON BIOAVAILABILITY
The notion that emulsion particle size affects the uptake of an active solubilized within a SEDDS formulation is frequently debated, and not surprisingly, any correlation made between droplet size and uptake is generally dependent on formulation chemistry and dissolution medium. The emulsion particle size is dynamic, becoming finer throughout the lipolysis process, and a lipid-based system undergoes intricate physiological progressions having many variables.14 Therefore, concluding particle size by itself correlates to bioavailability can be misleading.
A potential reason for this common misunderstanding could be a historical view that small particle size is directly correlated to consistent and predictable dispersions. This idea was perpetuated by the 1994 study comparing Sandimmune, a cyclosporin soft gel capsule that formed a crude emulsion in the gut, to the second-generation Neoral formulation, a soft gel capsule containing a cyclosporin SMEDDS formulation. The latter self-microemulsified upon contact with gastric fluid, while the former required dispersion by digestive enzymes to emulsify. This study concluded the microemulsifying nature of Neoral simplified dispersion, leading to more consistent dispersion profiles.15
A more recent study highlighted the difficulty correlating in-vitro dispersion size to in-vivo uptake. It examined in-vivo performances of different self-emulsifying Danazol formulations with varying oil/surfactant ratios resulting in a range of emulsion droplet sizes. In this study, uptake decreased with decreasing particle size, while uptake increased with course emulsions. The emulsion formulas contained three times as much digestible Maisine CC oil (glyceryl monolinoleate) compared to the non-digestible microemulsion containing increased percentages of Cremophor EL and ethanol. The alternative conclusion was therefore digestibility is a better predictor of bioavailability.16
In-vitro lipolysis is a screening technique used to simulate this mechanism. In-vitro lipolysis has been well documented to be more representative of a lipid formulation’s ability to maintain an active’s solubility in-vivo as the model accounts for the presence of enzymes that represent the dynamic conditions that occur in lipid metabolism.17
LIPIDS ARE STABLE & EASY TO HANDLE
As with other excipients, there are potential pitfalls that can occur when handling lipid-based materials. This has led to a misconception that lipids are unstable and difficult to work with, especially in terms of degradation via oxidation or hydrolysis. Oxidation can occur when sensitive moieties are exposed to oxygen. Oxidation reactions form peroxides from these moieties that propagate into additional peroxides that then generate unstable systems. Hydrolysis occurs when ester bonds are exposed to environmental moisture and react with water, cleaving these bonds and leading to formation of free fatty acids and reduction in ester content.
It’s important to keep in mind these sensitivities are prevalent in many excipient chemistries to a far greater extent especially in terms of propensity to oxidation. The omnipresence of oxygen-sensitive materials in pharmaceutical products is a testament to the manageability of oxidative challenges in drug development. Lipid-based excipients are quite easy to work with and can bring drug delivery solutions that greatly outweigh their controllable challenges. It is important to keep in mind when researching potential degradation pathways for lipids that they are often studied using forced experimental conditions.18 Oxidation and hydrolysis are avoidable using simple preventative measures in terms of storage and formulation in a pharmaceutical setting.
Lipid-based materials should be stored in sealed containers under inert conditions by purging headspace with nitrogen between uses. When melting and processing, dry heat sources should be used. Water baths are to be avoided when applying heat to melt and mix the formulation components. Instead, laboratory microwave ovens can be used to heat in short intervals. Commercial packs can be heated in ovens, hot boxes, or drum warmers.
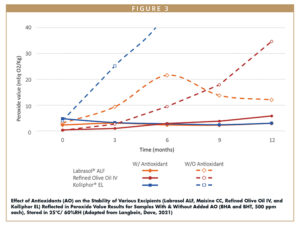
To avoid potential oxidation from occurring during the formulation process, it is highly recommended to incorporate antioxidants into formulations during the initial stages of development. In a recent white paper, evolution of peroxide value, a critical stability parameter and indicator of oxidation of lipid excipients, was examined with and without antioxidants BHT (butylated hydroxyanisole) and BHA (butylated hydroxytoluene). In Figure 3, one can see that in each case, peroxide value evolved at a significantly slower rate in the presence of antioxidants.19
DOSING IN PRECLINICAL STUDIES
Lipid vehicles are ideal candidates for preclinical screening studies due to their long history of use and ability to achieve high exposure due to bioavailability-enhancement mechanisms. A common misconception is lipid-based vehicles cause inevitable gastric irritation, tolerability issues, and toxicity in certain animal models. This belief stems from labs dosing subjects at improper levels or using poor dosing techniques and not from the lipids themselves.
As an example, rodents suffering from gastric irritation or emesis after having been orally dosed with Labrasol often arises when drugs are dosed neat in Labrasol at maximum recommended volumes for rodents. The rodents are unable to effectively metabolize lipids at this volume, which leads to negative effects. To avoid this, determine the saturation solubility of the drug in a volume of Labrasol for dosing of your target drug load to assess whether the required volume of Labrasol is within no observed adverse effect limits. If the required use level is too high, reduce the amounts of Labrasol in combination with other vehicles to achieve required solubility targets while remaining within known safety limits.
Dose-escalation studies that push boundaries of preclinical formulations should always refer to safety limit evaluations of single lipid excipients in the test species. Like other surfactant chemistries, lipid-based surfactants can cause more gastric irritation due to their surface-active interactions with the gut lining than simple glycerides; therefore, recommended safety dosing levels should be adhered to. The lipid formula can be optimized to achieve uptake targets at later stages and tested for safety once safety limits of the active have been well characterized. Gattefossé has extensive safety studies available on our excipients to assist in optimizing early stage tox formulations.
Having had a high number of lipid-based oral drugs approved by the FDA and available in the market shows how well lipid vehicles are tolerated when developed by formulators who dose the materials correctly. A 2017 review includes 36 examples of FDA-approved lipid-based formulations, including Absorica and Xtandi, which contain high levels of polyoxylglyceride-based surfactants, demonstrating lipid systems have withstood not only preclinical safety testing but also vigorous clinical human trials.1
SUMMARY
Bioavailability enhancement and processing flexibility are just a few of the facilities lipid-based excipients, due to their diverse physicochemical features and functionalities, can provide. Their chemistries have well-defined tox and safety profiles, and they offer multifunctionality, such as solubility, penetration, and bioavailability enhancers. They are also straightforward, easy-to-use, and compatible with solvent-free processes.
LBDDS can advance formulations from early stages through to commercialization when basic dosing recommendations and handling protocols, some of which have been previously discussed, are followed, thereby reducing costs and minimizing the time to market.
Having been used for decades on a global scale, LBDDS are viable platforms that should certainly be adopted into the formulator’s toolbox for use as key components of pharmaceutical product development.
REFERENCES
- Savla R, Browne J, Plassat V, Wasan KM, Wasan EK. Review and analysis of FDA approved drugs using lipid-based formulations. Drug Dev Ind Pharm. 2017 Nov;43(11):1743-1758. doi: 10.1080/03639045.2017.1342654.
- O’Driscoll CM. Lipid-based formulations for intestinal lymphatic delivery. Eur J Pharm Sci. 2002 Jun;15(5):405-15. doi: 10.1016/s0928-0987(02)00051-9.
- El-Laithy HM, Basalious EB, El-Hoseiny BM, Adel MM. Novel self-nanoemulsifying self-nanosuspension (SNESNS) for enhancing oral bioavailability of diacerein: Simultaneous portal blood absorption and lymphatic delivery. Int J Pharm. 2015 Jul 25;490(1-2):146-54. doi: 10.1016/j.ijpharm.2015.05.039. Epub 2015 May 19.
- McCartney Fiona et al. Labrasol® is an efficacious intestinal permeation enhancer across rat intestine: Ex vivo and in vivo rat studies. Journal of Controlled Release 310 (2019) 115–126.
- McCartney, Fiona et al. Labrafac™ MC60 acts as an intestinal permeation enhancer in isolated rat colonic and jejunal mucosae in the Ussing chambers. University College Dublin as funded by Gattefossé. Poster. CRS. 2022.
- Ukai H, Iwasa K, Deguchi T, Morishita M, Katsumi H, Yamamoto A. Enhanced Intestinal Absorption of Insulin by Capryol 90, a Novel Absorption Enhancer in Rats: Implications in Oral Insulin Delivery. Pharmaceutics. 2020 May 18;12(5):462. doi: 10.3390/pharmaceutics12050462.
- Lin Y, Shen Q, Katsumi H, Okada N, Fujita T, Jiang X, Yamamoto A. Effects of Labrasol and other pharmaceutical excipients on the intestinal transport and absorption of rhodamine123, a P-glycoprotein substrate, in rats. Biol Pharm Bull. 2007 Jul;30(7):1301-7. doi: 10.1248/bpb.30.1301.
- Dubray O, Jannin V, Demarne F, Pellequer Y, Lamprecht A, Béduneau A. In-vitro investigation regarding the effects of Gelucire® 44/14 and Labrasol® ALF on the secretory intestinal transport of P-gp substrates. Int J Pharm. 2016 Dec 30;515(1-2):293-299. doi: 10.1016/j.ijpharm.2016.10.012.
- Jannin V, Cuppok Y. Hot-melt coating with lipid excipients. Int J Pharm. 2013 Dec 5;457(2):480-7. doi: 10.1016/j.ijpharm.2012.10.026. Epub 2012 Oct 23.
- Rosiaux Y et al. Fundamental differences in hot melt extrusion between polymers and solid lipid excipients. Poster. AAPS. 2015.
- El-Say KM, Ahmed TA, Ahmed OAA, Hosny KM, Abd-Allah FI. Self-Nanoemulsifying Lyophilized Tablets for Flash Oral Transmucosal Delivery of Vitamin K: Development and Clinical Evaluation. J Pharm Sci. 2017 Sep;106(9):2447-2456. doi: 10.1016/j.xphs.2017.01.001. Epub 2017 Jan.
- Gattefossé SAS Technical Center of Excellence. Unpublished data. Report on in vitro lipolysis of Cinnarizine. 2018.
- Larsen A, Holm R, Pedersen ML, Müllertz A. Lipid-based formulations for danazol containing a digestible surfactant, Labrafil M2125CS: in vivo bioavailability and dynamic in vitro lipolysis. Pharm Res. 2008 Dec;25(12):2769-77. doi: 10.1007/s11095-008-9641-0. Epub 2008 Jul 1.
- Vithani K, Jannin V, Pouton CW, Boyd BJ. Colloidal aspects of dispersion and digestion of self-dispersing lipid-based formulations for poorly water-soluble drugs. Adv Drug Deliv Rev. 2019 Mar 1;142:16-34. doi: 10.1016/j.addr.2019.01.008.
- Kovarik JM, Mueller EA, van Bree JB, Tetzloff W, Kutz K. Reduced inter- and intraindividual variability in cyclosporine pharmacokinetics from a microemulsion formulation. J Pharm Sci. 1994 Mar;83(3):444-6. doi: 10.1002/jps.2600830336.
- Cuiné JF, Charman WN, Pouton CW, Edwards GA, Porter CJ. Increasing the proportional content of surfactant (Cremophor EL) relative to lipid in self-emulsifying lipid-based formulations of danazol reduces oral bioavailability in beagle dogs. Pharm Res. 2007 Apr;24(4):748-57. doi: 10.1007/s11095-006-9194-z.
- Bayarri M, Akkermans A, Teles H. Relationship between in vitro lipolysis release and in vivo performance of lipid-based drug delivery systems for a BCS class II compound. Patheon Softgels as part of Thermo Fisher. Poster. AAPS. 2018.
- Musakhanian J, Rodier JD, Dave M. Oxidative Stability in Lipid Formulations: a Review of the Mechanisms, Drivers, and Inhibitors of Oxidation. AAPS PharmSciTech. 2022 May 20;23(5):151. doi: 10.1208/s12249-022-02282-0. Erratum in: AAPS PharmSciTech. 2022 Jun 14;23(5):165.
- Langbein D, Dave M. Key Considerations for Stabilizing Oxidation-Prone Lipid-Based Drug Delivery Systems. White Paper. American Pharmaceitical Review. June 2021. https://www.americanpharmaceuticalreview.com/Featured-Articles/577086-Key-Considerations-for-Stabilizing-Oxidation-Prone-Lipid-Based-Drug-Delivery-Systems/.

Rollie Fuller is Technical Service Coordinator at Gattefossé USA, where he is responsible for provision of technical, quality, and regulatory information to customers. He joined the Technical Service Department in 2019 and has since become an integral part of in internal and external tech support initiatives. He earned his BSc in Chemical and Biomolecular Engineering from New York University in 2017.

Ron Permutt is Senior Director, Pharmaceutical Division, at Gattefossé USA, where he oversees the pharmaceutical sales, technical, marketing, and laboratory operations in Paramus, NJ. Prior to joining Gattefossé in 1999, he worked at Cerestar, developing cyclodextrin business in the northeastern United States and prior to that as Plant Research Engineer at JM Huber Ink Division. He earned his MBA from Fairleigh Dickinson University, NJ, and his BSc in Chemical Engineering from Northwestern University, IL.
Total Page Views: 7225