Issue:March 2020
INTRATUMORAL DELIVERY - Combining Local & Systemic Treatments: Could Immuno-Oncology Finally Enable Local Intratumoral Delivery?
INTRODUCTION
Tip O’Neill, former US Speaker of the House, is associated with the phrase “all politics is local.” Like politics, all tumors are local. However, visible tumors may only be part of one’s cancer; so unlike politics, cancer can be both local and systemic.
Surgery, often with chemotherapy, is the main treatment method for solid tumors. Immunotherapy, also referred to as immune-oncology or IO, is a new approach generating much excitement. IO harnesses the immune system and offers patients the potential for long-term remission. Many oncologists are now using the term “cure,” which was previously unheard of in relation to cancer.
Unfortunately, recent clinical data have clouded the blue sky promise of IO. Data suggests that the benefits of immunotherapy may be limited to “immunologic” or “hot” cancers, ie, those types in which immune cells can recognize the cancer. Yet, even in those cancers, immunotherapy treatment for many patients is inadequate. Could coupling IO with new local treatments, such as intratumoral drug administration, overcome IO’s current limitations? Are there intratumorally delivered products that could turn “cold” tumors “hot”? Let’s explore the long arc of cancer treatment and review the exciting potential of novel local approaches coupled with modern immunotherapy.
CANCER IN HISTORY
The human record of cancer is nearly as old as written language. A papyrus scroll from ancient Egypt (~1600 BCE) describes a process to remove tumors of the breast by cauterization.1 Throughout the centuries, many theories were put forward about the origins of cancer. At the beginning of the enlightenment, physicians began to seek the causes of cancer in a scientific manner.
LOCAL APPROACHES
Cut-It-Out: Surgery
Initially, the local nature of cancer dominated thought. In the mid-1700s, John Hunter began the era of modern surgery. Hunter saw that cancer was moveable and operated to remove tumors. The use of the microscope to study tissue also began during this period. Rudolf Virchow developed the concept of “pathological processes.” His application of cell theory explained the effects of disease in the organs and tissues of the body.2 This knowledge led to the modern concept that surgery is most effective if the cancer is caught early and has not spread. Finally, scientists began to think of cancer as both a local and systemic disease.
Beam-It-Out: Radiation
Wilhelm Conrad Röntgen discovered X-rays in 1895.3 One year later, those powerful beams were used by Emil Herman Grubbe to treat a patient with breast cancer.4 Again, a new local treatment idea took hold. Radiotherapy (RT), also known as radiation therapy, is a local treatment that uses high-energy rays or radioactive substances to damage tumoral cells to halt their growth and division. RT is now quite common with about two-thirds of all cancer patients receiving the therapy as a unique treatment or as part of a more complex therapeutic protocol.5 Unfortunately, during RT, normal cells, especially those that divide frequently, may also be damaged and killed. Radiation is not an effective treatment modality in metastatic settings for many tumor types, especially when tumors are large and deep in the body.
Melt or Freeze Away: Ablation
Ablation is a minimally invasive local treatment method for solid cancers. Various methods of imaging are used to guide and position a probe into the tumor. This requires only a tiny hole to reach the cancer. A generator attached to a probe within the cancer “burns” or “freezes” the tumor. The effectiveness of ablation technique in treating cancer depends on two things: the size of the tumor and its accessibility. In general, for tumors three centimeters or less and easily accessible, the technique can work well.6 There are only a limited number of tumors that can be treated, and there is no systemic component for ablation techniques. The approach is mainly used in treating small liver lesions.
SYSTEMIC APPROACHES
Poison Potions: Chemotherapy, Targeted Therapy, Antibodies
When the cancer is beyond the local environment, even if only a few cells are found in the neighboring lymph nodes, systemic therapies are often added to local methods or used alone. At the start of the 20th century, Paul Ehrlich began developing drugs to treat infectious diseases. Ehrlich first used the term “chemotherapy” and defined the word as the use of chemicals to treat disease.7 One of the first chemicals tested against cancer was a derivative of an agent used to kill soldiers in World War I. Today’s molecular arsenal includes hundreds of compounds that, once inside the cancer cell, disrupt various processes to kill the cells. The problem is that healthy fast-dividing cells, such as those in the scalp or gut, also absorb these agents, resulting in severe toxicities. Targeted agents acting like a guided missile are highly specific to various tumor types and have shown great promise. Unfortunately, there are not many cancers for which patients have the correct molecular profile for these agents to work. When the agents do work, they work well, almost miraculously. Though often, the tumor mutates and the drugs lose effectiveness. Despite tremendous funding and initial enthusiasm about molecular targeting drugs, such as kinases or inhibitors of growth factor receptors, long-term patient outcomes are frequently disappointing.8 Often a patient will suffer multiple lines of drug therapy with severe side effects and diminishing rates of return. Once a patient with metastatic disease fails two or more lines of drug therapy, their odds of long-term survival are significantly reduced.
Harness the Defenses: Immunotherapy
In October 2018, Dr. James Allison and Dr. Tasuku Honjo were awarded the Nobel Prize in Physiology or Medicine for their groundbreaking cancer research. Allison studied a protein on the surface of T cells called CTLA-4, discovering that it inhibits immune cells. Honjo and his colleagues were studying another T-cell protein called PD-1, or programmed cell death protein-1, which they identified in 1992.9 The antibody products that affect these immune cell proteins have become blockbuster drugs and today are the basis for the majority of clinical cancer research. The idea of using the immune system to fight cancer is not new. Willian Bradley Coley in the early 1900s began to experiment with using a mixture of killed bacteria as a treatment for cancer. Even further back in 2600 BCE, the Pharaoh Imhotep deliberately infected his tumors to attack his cancer. Modern medicine has now come full circle back to the Egyptians. One of the biggest challenges of IO is recognition by the immune system. Cancer is derived from a patient’s healthy tissue. Essentially the immune system cannot easily distinguish cancer from healthy cells. Because the immune system is programmed not to attack a person’s tissue, the cancer can grow unimpeded. Some tumor biomarkers such as the percent expression of PD-L1 protein or a tumor’s mutational burden (TMB) values are indicative of who will respond. Patients fortunate enough to have tumors recognizable to the immune system are those most likely to benefit. Despite their promise and hype, drugs that act on the immune system currently only benefit a fraction of cancer types and patients.
REGIONAL ADMINISTRATION
Surgery and chemotherapy remain the leading workhorses of cancer treatment. If cytotoxic agents can kill cancer but cause systemic side effects, why not administer these drugs locally? Local delivery could kill the tumors, leave healthy tissue unharmed, minimize off-target toxicities, and be less invasive than surgery. Even though there may not be a systemic benefit of having “surgery in a bottle,” for cancers confined in a region or for single tumors local administration should be of benefit. This win-win idea is old and has been proposed since the discovery of these killing agents; however, success has only been marginal.
There are two main concepts of local drug delivery to treat cancer. The first is delivery solely to the specific affected organ or part of the body, ie, regional delivery. Physicians currently use regional delivery in settings such as the liver, limbs, peritoneum, localized areas of the skin, and even in some central nervous system (CNS) settings. One of the most common modalities is in the liver and is known as trans-arterial chemo-embolization (TACE). The second approach is to be more precise by administering the drug directly into the tumors, ie, intratumoral delivery. Both regional and intratumoral delivery spare the majority of the body of the ill effects of chemotherapeutic agents.
The Challenges of Intratumoral Delivery
There are many cancers that originate deep in the body. To treat these tumors requires the aid of a visualization technique to determine where to place the injection needle. Fortunately, the recent development of computer aided tomography (CT) and easy-to-use ultrasound devices have made image guidance much more economical, precise, and practical.
Aside from imaging requirements, there are other problems that need to be solved for effective intratumoral delivery. The first is how to disperse drug throughout the tumor. Most potent agents are given systemically, and the drugs have thus been designed or formulated for the blood stream, which is an aqueous fluid. In general, cytotoxic drugs are hydrophilic and lipophilic compounds are challenging to formulate. Yet tumors often contain a high percentage of lipids, with some types as high as 30%.10 Aqueous formulations are not well absorbed into these tumors. If there is chemical incompatibility of the drug with the tumor, then thorough dispersion and good absorption may be difficult.
The second key issue is for penetration of the potent agents into the cancer cells. There are three main cell internalization routes a molecule can take; via a receptor, by endocytosis of a vacuole, or by diffusion. Receptor-based transport is mostly genetically determined. Without a sufficient number of receptors, internalization is limited. Additionally, endocytosis is inherently slow. Finally, if the agent is water-loving, it will be incompatible with the cancer cell membrane, and diffusion will be poor.
Throughout the years, there have been several attempts at delivering cytotoxic agents intratumorally. These technologies include delivery in nanoparticle formulations, use of retentive gels, vasoconstrictors, microwaves, electroporation, and many other schemes.11-14 None of these approaches adequately solved the dispersion and diffusion problems, and intratumoral dosing often failed to show benefit over systemic administration coupled with the local treatment.
Immunotherapies Revive the Potential of Intratumoral Delivery
With the advent of immunotherapy, intratumoral delivery has recaptured the interest of the biotech industry. In October 2015, the FDA approved Amgen’s Imlygic® (talimogene laherparepvec) for the treatment of localized melanoma.15 Also known as T-Vec, the drug is a modified form of the herpes virus dosed by direct intratumoral injection. Unfortunately, efficacy is not strong and sales of this drug are quite low. Several other companies have initiated clinical programs to test the intratumoral delivery of agents that can cause an immunological inflammation in the tumor microenvironment. These programs include intratumoral delivery of RIG-1, STING, and TLR9.16-18
Though the idea for intratumoral delivery to provide inflammation and antigen release is intriguing, these agents still need to be absorbed, retained, and dispersed in the tumor to be effective. Chemical compatibility between the drug formulation and the tumor is paramount.
A New Approach for Intratumoral Delivery
A new technology that can increase dispersion and diffusion of drugs in the tumor microenvironment is being tested in the clinic.19 The drug, INT230-6, contains cisplatin and vinblastine co-formulated with an amphiphilic agent that improves tumor dispersion and cancer cell penetration. A recent paper indicates the drug induces direct cancer cell death as well as immune activation. INT230-6 is also quite effective when combined with immunotherapies.20 The ability to attenuate the tumor in situ without damaging the cancer cell membranes may mean that INT230-6 has the potential to turn “cold” tumors “hot”, ie, increase immune cell recognition, which could solve a major challenge of immunotherapy. Early clinical results are promising.
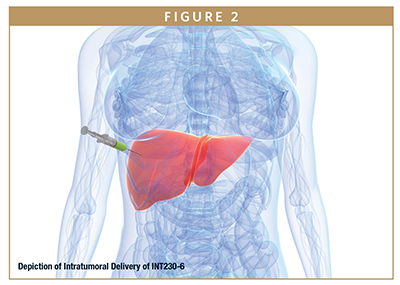
DISCUSSION
There are now multiple weapons against cancer, including modern surgery; high-tech radiation; sophisticated ablative techniques; novel compounds comprising small molecules, peptides, oligonucleotides, or proteins; and immunotherapies. Despite these armaments, over 606,000 Americans are expected to die from cancer in 2019.21 There is hope as the 5-year survival rates from 1975-1977 to 2008-2014 have gone from 49% to 69%. While there has been progress over the past 45 years, medicine is still a long way away from a cure for most cancers, especially late-stage disease.
CONCLUSIONS
There is often a long arc to fighting illnesses. Infectious diseases, such as bubonic plague, smallpox, and polio, killed or disabled hundreds of millions of people over the millennia. Today, in advanced countries, many of these dreaded diseases have been eradicated. Cancer too has a long and ancient history. We now understand that cancer is a local and systemic disease. New forms of local treatment, such as intratumoral dosing coupled with systemic immunotherapy, are being explored. If humanity is to win the fight against the Emperor of all Maladies a significant shift in treatment approaches beyond surgery with systemic chemo will be required.22 Whether replacing cutting and poisoning by local delivery and immune activation can be achieved, only time will tell. One thing is certain, given the perseverance and dedication of scientists and our exponentional growth in understanding the complexities of cancers, someday this disease too shall be tossed into the dustbin of history. Hopefully that day is near.
REFERENCES
- “The History of Cancer”. American Cancer Society. 2009.
- https://www.britannica.com/biography/Rudolf-Virchow.
- Rontgen WC Uber eine neue Art von Strahlen. Vorl¨aufige Mitteilung. Vol. 30. Sitzung: Sitzungsberichte¨der physikalisch-medicinischen Gesellschaft zu Wurzburg; 1985. pp. 132-141.
- Grubbe EH. Priority in the therapeutic use of X-rays. Radiology´ 1933;21:156-162.
- Gianfaldoni, S, et. al J Med Sci. 2017 Jul 25; 5(4):521-525.
- https://www.hopkinsmedicine.org/interventionalradiology/procedures/tumor/index.html.
- Vincent T. DeVita Jr. and Edward Chu, A History of Cancer Chemotherapy,DOI: 10.1158/0008-5472.CAN-07-6611. Published November 2008.
- Hiroshi Maeda and Mahin Khatamic, Clin Transl Med. 2018;7:11. Analyses of repeated failures in cancer therapy for solid tumors: poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs.
- https://www.the-scientist.com/news-opinion/james-allison-and-tasukuhonjo-win-nobel-prize-64879.
- Morio Yasuda, W.R. Bloor, Lipid Content of Tumors, J Clin Invest. 1932;11(4):677-682. https://doi.org/10.1172/JCI100442.
- Al-Ghananeem, Abeer, M., Intratumoral Delivery of Paclitaxel in Solid Tumor from Biodegradable Hyaluronan Nanoparticle Formulations, AAPS Pharm SciTech. 2009 Jun;10(2):410-417.
- Fakhari J., Subramony, A., Engineered in-situ depot-forming hydrogels for intratumoral drug delivery, Journal of Controlled Release Volume 220, Part A, 28 December 2015, Pages 465-475.
- Conley FK, Luck EE, Brown DM, Response of murine tumors to matrixassociated cisplatin intratumoral implants. NCI Monograph. 1988;(6):137-40.
- Canton DA1, Shirley S., Melanoma treatment with intratumoral electroporation of tavokinogene telseplasmid (pIL-12, tavokinogene telseplasmid), Immunotherapy. 2017 Dec;9(16):1309-1321.
- https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapyproducts/imlygic-talimogene-laherparepvec.
- Middleton M.R, Wermke, M., ESMO Conference October 2018 Munich, Phase 1/2, Multicenter, Open-Label Study of Intratumoral/Intralesional Administration of the Retinoic Acid–Inducible Gene I (RIG-I) Activator MK-46.
- Bernstam, F.M., Sandhu, Phase Ib study of MIW815 (ADU S100) in combination with spartalizumab (PDR001) in patients advanced/metastatic solid tumors or lymphomas (NCT03172936). ASCO 2019.
- Fakhari A1, Nugent S2, Thermosensitive Gel-Based Formulation for Intratumoral Delivery of Toll-Like Receptor 7/8 Dual Agonist, J Pharm Sci. 2017 Aug;106(8):2037 2045.
- El-Khoueiry, A., Thomas, J, INT230-6, a novel intratumoral (IT) formulation demonstrated a favorable safety profile during injections into a variety of refractory deep and superficial tumors with evidence of tumor regression and immune activation, ASCO 2019 Abstract 2602.
- Bloom, A., Bender L. Intratumorally delivered formulation, INT230-6, containing potent anticancer agents induces protective T cell immunity and memory. OncoImmunology Volume 8, 2019 Issue 10.
- Cancer Statistics Data CA Cancer J Clin 2018;00:00-00.
- See book with this title by Siddhartha Mukherjee

Lewis H. Bender is Founder and CEO of Intensity Therapeutics, a clinical-stage biotechnology company pioneering a novel, immune-based approach to treat solid tumor cancers through direct injection of the company’s proprietary therapeutic agents. He has more than 26 years of leadership in the biopharmaceutical industry, taking products leveraging novel drug delivery techniques from discovery through Phase 3 development and partnering with major pharmaceutical and biotechnology companies. Previously, he was the CEO of Interleukin Genetics (IG) and held numerous positions at Emisphere Technologies, Inc., including CEO and VP of Business Development & Manufacturing. He earned his SB and SM in Chemical Engineering from the Massachusetts Institute of Technology (MIT), his MBA from the Wharton School of the University of Pennsylvania (UPenn), and his MA in International Studies, also from UPenn.
Total Page Views: 6708










