Issue:April 2023
INFLAMMASOME INHIBITORS - 21st Century Miracle Drugs: Spotlight on Clinical NLRP3 Inflammasome Inhibitors
INTRODUCTION
In 1860, Henry Wadsworth Longfellow immortalized America’s most famous early detection warning system with the hanging of lanterns in the tower of the Old North Church and the subsequent midnight ride of Paul Revere to warn colonists of the approaching British army. “One if by land, and two if by sea” the phrase coined by Henry W. Longfellow is a reference to the secret signal orchestrated by Revere.1
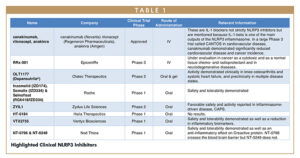
The Paul Revere of the innate immune system, which constitutes the first line of defense against non-self-pathogens, is known as the NLRP3 inflammasome. This inflammasome senses danger and responds through the activation of genes that encode IL-1beta, an inflammatory signaling molecule. IL-1beta sounds the alarm and puts the immune system on hyperalert status. The inflammasome also initiates a cellular self-destruct sequence in which a protein called gasdermin D pokes holes in the macrophages from the inside out, leading to release of IL-1beta and to a type of cell death called pyroptosis.
This is beneficial — to a point — and only to a point. Past a certain point, inflammation is a self-sustaining chain reaction in which inflammation leads to damage, damage leads to more inflammation, and more inflammation leads to more damage, etc, until a persistent inflammatory state is maintained. It is no surprise, then, that hyperactive NLRP3 inflammasomes are linked to an enormous range of diseases, including Alzheimer’s, atherosclerosis, inflammatory bowel disease, nonalcoholic steatohepatitis (NASH), and Parkinson’s. One strategy to slow or stop the progression of these diseases is to inhibit the inflammasome.
Several specific inflammasome inhibitors that have entered the clinic are briefly reviewed below, although clinical data from them is not always accessible.
RRX-001
The most clinically advanced of the direct NLRP3 inflammasome inhibitors, RRx-001, a parenterally administered small molecule from EpicentRx that crosses the blood brain barrier, is in Phase 3 trial for the treatment of third line and beyond small cell lung cancer (SCLC) and in a late-stage trial for the treatment of severe oral blistering or mucositis from radiation and chemotherapy in head and neck cancer and on the critical path for approval as a radioprotectant in case of nuclear war.2 Because it crosses the blood brain barrier, RRx-001 is also under study in Parkinson’s Disease, Amyotrophic Lateral Sclerosis (ALS)/motor neuron disease (MND), and Alzheimer’s Disease having been awarded grants as an inflammasome inhibitor by the Michael J Fox Foundation (MJFF) and other funding organizations for neurodegenerative diseases.3
OLT1177 (DAPANSUTRILE®)
This is a highly selective inhibitor of NLRP3 in oral and gel form from Olatec Therapeutics, which has completed a Phase 2 clinical trial for osteoarthritis of the knee. In a Phase 2 clinical trial for gout, OLT1177 was reported to reduce joint pain comparably to NSAIDs and prednisolone, and in a Phase 1 trial for systolic heart failure left ventricular ejection fraction was improved.4,5 OLT1177 is in an ongoing Phase 2 trial for Schnitzler’s syndrome, a rare inflammasome-driven autoinflammatory disease. It is also under preclinical investigation in Alzheimer’s Disease, autoimmune encephalomyelitis, myocardial ischemia, and arthritis.6 No safety issues have been reported.
DFV890 (IFM-2427)
Acquired by Novartis in 2019, IFM Tre developed an oral NLRP3 inflammasome, IFM 2427, later renamed DFV890 after the Novartis acquisition, which has reportedly completed Phase 1 safety trials. DFV890 is in Phase 2 trials for the treatment of familial cold autoinflammatory syndrome (CAPS) and knee osteoarthritis. No results are available.
CANAKINUMAB (ILARIS), RILONACEPT (ARCALYST) & ANAKINRA (KINERET)
While not pure inflammasome inhibitors, the parenterally administered biologics canakinumab, rilonacept, and anakinra deserve honorable mention as blockers of IL-1 because this inflammatory cytokine is a product of NLRP3 inflammasome activation. All are approved by the FDA for the treatment of cryopyrin-associated periodic syndromes (CAPS), an autoinflammatory disease that results from a mutated, hyperactive inflammasome. Anakinra is also approved for the treatment of moderate-to-severe rheumatoid arthritis, while canakinumab is approved for juvenile idiopathic arthritis [JIA]. Canakinumab (Ilaris) completed a large 10,000+ patient Phase 3 clinical trial named CANTOS in cardiovascular disease. CANTOS demonstrated not only significantly reduced recurrent cardiovascular events but also, more unexpectedly, a significantly lower total cancer mortality. However, the canakinumab-treated group had an increased risk of fatal infection and sepsis.7
INZOMELID (IZD174), SOMALIX (IZD334) & SELNOFLAST (RG6418/IZD334)
Inflazome, a joint spin-out from the University of Queensland in Australia and Trinity College Dublin in Ireland, which Roche acquired in 2020, tested two oral NLRP3 inhibitors in Phase 1 trials. These were Inzomelid (IZD174), a brain-penetrant molecule, and peripherally restricted Somalix (IZD334), which were evaluated in a Phase 1 and a Phase 1B trial in CAPS. Except for safety and tolerability, which were acceptable, no results from these trials are available, but Phase 2 trials are reportedly planned.8 Selnoflast (RG6418/IZD334), another inflammasome inhibitor, reportedly finished a Phase 1 trial in CAPS patients but data is not available.
ZYIL1
This potent orally administered NLRP3 inhibitor from Zydus Lifesciences completed a Phase 2 proof-of-concept trial in CAPS patients with documented flare ups. In addition to favorable safety and tolerability, sustained clinical improvement was reportedly demonstrated.9
HT-6184
An orally administered small-molecule inhibitor of NEK7 and NLRP3 pathway from Halia Therapeutics, HT-6184 is in an ongoing Phase 1 trial with healthy volunteers.10
VTX2735
A peripheral orally administered NLRP3 inhibitor from Ventyx Biosciences, VTX2735 completed a Phase 1 trial in healthy volunteers. Safety and tolerability were demonstrated, and dose-related suppression of the inflammatory cytokine IL-1β and high sensitivity C-reactive protein (hsCRP) concentrations were observed relative to placebo. A Phase 2 trial in CAPS is planned.11
NT-0796 & NT-0249
These oral NLRP3 inhibitors from NodThera completed a Phase 1 trial in which safety and tolerability were demonstrated as well as an anti-inflammatory effect via reduction of C-reactive protein. NT-0796 crosses the blood brain barrier, whereas NT-0249 does not.12
DISCUSSION
Inflammation has been compared to a double-edged sword or a Janus head with two faces that helps as well as harms, depending on the context.13,14 On the one hand, inflammation is a protective response that countenances the use of lethal force to shorten biological “battles” with infectious agents like bacteria, viruses, fungi, and parasites. On the other hand, collateral damage to healthy tissues is the inevitable byproduct of the indiscriminate release of oxidants and free radicals from activated white blood cells that are pressed into service during inflammation. The longer an inflammatory response persists the more widespread the damage to bystander cells and tissues results; this is the reason for the existence of several molecular “off switches” like the cytokines, interleukin 10 (IL-10), and transforming growth factor-β (TGF-β), which are released to downregulate inflammation and to prevent excessive damage. Inflammation is “good” when it remains space-, time- and intensity-limited, and “bad” when it is systemic, chronic, or dysregulated.
Chronic inflammation, which occurs when acute inflammation fails to properly resolve, underlies many important diseases, including angina, arthritis, inflammatory bowel disease, and even aging. However, it is important to emphasize inflammation, whether acute or chronic, is not always synonymous with infection because dead cells, ischemia, and irritant particles, including crystals like cholesterol, minerals like silica, and protein aggregates (ie, proteins that clump together) like beta-amyloid in Alzheimer’s, can also cause it. This so-called sterile or pathogen-free inflammation is particularly important because of its association with age-related disorders and diseases including frailty.
Textbooks of medicine describe thousands of separate diseases, all with different causes, manifestations, symptoms, clinical courses, and treatment options, which suggests that an equal number of distinct responses and mechanisms are at work. However, regardless of the disease entity, one response/mechanism is practically invariant: increased blood supply to the site where the injurious agent (ie, pathogens, dead cells, irritant particles, or toxins) is located with the accumulation and activation of white blood cells there, such as neutrophils, macrophages, Langerhans cells, and dendritic cells to eliminate it. This is experienced as the five cardinal signs of inflammation, ie, redness, swelling, heat, pain, and loss of function, the purpose of which is to eliminate with prejudice any and all perceived threats to the integrity of the host tissues.15 In the process, the collateral damage wrought by inflammation to normal tissues and organs may initiate, exacerbate, or perpetuate dysfunction and disease, especially when the inflammatory response is unregulated and uncontrolled.
SUMMARY
Traditionally, pharmaceutical development is based on the “magic bullet” concept of “one drug, one target, one disease.” However, with the growing realization that chronic inflammation lies at the center of many, if not all, diseases, it is possible to envision inflammasome inhibitors, which reduce or prevent inflammation, as near-universal treatment panaceas. If this sounds farfetched or overstated, consider that OLT1177 and RRx-001, the most clinically advanced of the direct inflammasome inhibitors, have between them demonstrated activity, mostly preclinical, in more than 50 different disease states.
To best knowledge, the only other pharmaceutical class that compares to inflammasome inhibitors in terms of broadness of potential application is glucocorticoids, such as hydrocortisone, prednisone, and dexamethasone, which, having been initially hailed as “wonder” or “miracle drugs” when they were first introduced in the 1950s, are still used today in almost every area of medicine to treat or manage acute and chronic inflammation.16,17 Different than glucocorticoids, however, which are associated with a range of side effects, depending on dose and length of use, such as hyperglycemia, weight gain, depression, glaucoma, infection, edema, and hypertension, the inflammasome inhibitors RRx-001, OLT1177, DVF890, Inzomelid, Somalix, and Selnoflast, ZYIL1, VTX2735, HT-6184, NT-0796 and NT-0249 have so far demonstrated a favorable safety profile.18
This suggests the possibility of their use not only as single agents, but also in combination with glucocorticoids, non-steroidal anti-inflammatory drugs (NSAIDs), IL-1 blockers, or other inflammasome inhibitors to prevent and treat some diseases and hopefully, in an absolutely best-case scenario, most or even all of them.
REFERENCES
- Riaud X. Paul Revere (1735-1818), the “midnight rider”. Dent Hist. 2009 Jan;(49):33-40. V. Klück, et al. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol., 2 (2020), pp. e270-e280
- Oronsky B, Reid TR, Larson C, Caroen S, Quinn M, Burbano E, Varner G, Thilagar B, Brown B, Coyle A, Ferry L, Abrouk N, Oronsky A, Scribner CL, Carter CA. REPLATINUM Phase III randomized study: RRx-001 + platinum doublet versus platinum doublet in third-line small cell lung cancer. Future Oncol. 2019 Oct;15(30):3427-3433.
- https://www.prnewswire.com/ news-releases/epicentrx-awarded-grant-from-the-michael-j-fox-foundation-to-evaluate-therapeutic-potential-of-rrx-001-in-parkinsons-disease-301584891.html
- G.F. Wohlford, et al. Phase 1B, randomized, double-blinded, dose escalation, single-center, repeat dose safety and pharmacodynamics study of the oral NLRP3 inhibitor dapansutrile in subjects with NYHA II-III systolic heart failure. J. Cardiovasc. Pharmacol., 77 (2020), pp. 49-60
- http://www.olatec.com/patients.html
- https://pipelinereview.com/ index.php/2022090781551/Small-Molecules/Zydus-announces-positive-Phase-2-Proof-of-Concept-of-NLRP3-inhibitor-ZYIL1-in-patients-with-Cryopyrin-Associated-Periodic-Syndrome-CAPS.html
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017 Sep 21;377(12):1119-1131.
- https://www.businesswire.com/ news/home/20200326005102/en/Inzomelid-completes-Phase-I-studies-and-shows-positive-results-in-the-treatment-of-Cryopyrin-Associated-Periodic-Syndrome-CAPS
- https://zydususa.com/
- https://www.clinicaltrialsarena.com/ news/halia-doses-trial-subject/
- https://drug-dev.com/ventyx-biosciences-announces-positive-topline-phase-1-data-for-its-peripheral-nlrp3-inhibitor/.
- https://www.prnewswire.com/ news-releases/nodthera-announces-positive-phase-1-study-readouts-for-the-nlrp3-inflammasome-inhibitors-nt-0796-and-nt-0249-301629146.html
- Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994 Dec;56(6):672-86.
- Wang X, Feuerstein GZ. The Janus face of inflammation in ischemic brain injury. Acta Neurochir Suppl. 2004;89:49-54.
- Punchard NA, Whelan CJ, Adcock I. The Journal of Inflammation. J Inflamm (Lond). 2004 Sep 27;1(1):1.
- Mirzayan MJ, Goessling T, Huefner T, Krauss JK. Subacute steroid-induced paraparesis: surgical treatment of a devastating “invisible” side effect. Eur Spine J. 2012 Jun;21 Suppl 4(Suppl 4):S542-4.
- Chalmers JR, Axon E, Harvey J, Santer M, Ridd MJ, Lawton S, Langan S, Roberts A, Ahmed A, Muller I, Long CM, Panda S, Chernyshov P, Carter B, Williams HC, Thomas KS. Different strategies for using topical corticosteroids in people with eczema. Cochrane Database Syst Rev. 2019 Jun 19;2019(6):CD013356.
- Yasir M, Goyal A, Sonthalia S. Corticosteroid Adverse Effects. [Updated 2022 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK531462/

Dr. Bryan Oronsky serves as EpicentRx’s Chief Development Officer and combines first-hand clinical experience with 17 years of pharmaceutical development experience. Before joining EpicentRx, he worked as a Medical Officer at Intarcia Therapeutics. He has published more than 100 peer-reviewed scientific articles, reviews, and book chapters. He is also co-inventor of 10 issued patients and more than 50 pending patents in the biomedical and device fields. He was educated at Princeton University, Catholic University in Belgium, and University of Miami Medical School.
Total Page Views: 13774