Issue:April 2013
IMPURITIES DETECTION - Impurities Detection in Pharmaceuticals
INTRODUCTION
As regulations on pharmaceuticals develop lower limits as for impurities, detection methods must become more sensitive and selective. Increasingly, it is important to detect the impurities not only in the active ingredient but in the final formulation. Thermal methods, FTIR, and GC/MS, are all used in this. For inorganics like traces of heavy metals, ICP-MS is an important tool. In this article, we focus on the detection of residual solvents from processing and small levels of contaminates leached from containers.
RESIDUAL SOLVENT DETECTION BY EGA
Thermogravimetric analysis (TGA) is a commonly used technique i pharmaceutical research and quality labs to investigate residual solvents in formulations. Pharmaceutical samples often show weight losses associated with the loss of solvent/water, desolvation, or decomposition of the sample. This information is then use to assess the purity and stability of the material and its suitability for use. The TGA gives a quantitative measure of mass lost from the sample, but it does not provide information on the nature of the products that are lost from the sample often required for complete characterization.
Several techniques exist to address this and are collectively known a Evolved Gas Analysis. The most common is the combination of a TGA with FTIR, referred to as TG-IR. An example would be a Pyris 1 TGA coupled to a Frontier FTIR running on Timebase software, which allows importation of the TGA data into the IR software. This technique allows one to determine what materials come off of the sample at which temperature within the limits of IR’s sensitivity and to map it as a function of temperature. One also has to deal with confounding affects and overlapping peaks. More sensitivity and resolution can be obtained by moving to a TG-MS system. Replacing the FTIR with a mass spectrometer (MS) allows for both increased sensitivity and improved resolution.
COUPLING TGA TO GC/MS
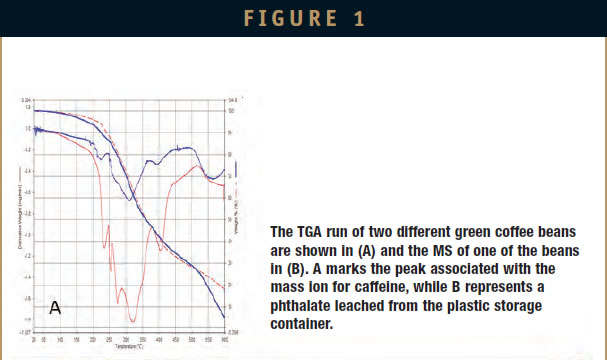
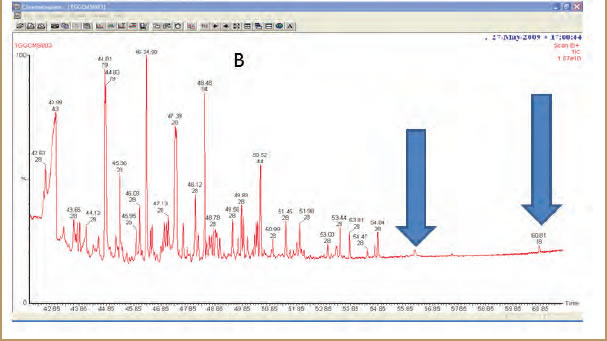
Even more resolution can be obtained by coupling a gas chromatographmass spectrometer (GC/MS) like the Clarus SQ 8 to a TGA. While this technique allows us to bring the full power of GC/MS to identify evolved materials, it requires collection of the gases. Several techniques exist to do this, including trapping materia on a column, gas collection loops, and use of Swafer technology. The downside is that we lose the temperature or time relationship to what was coming off the TGA. As the time/temperature evolution of the compound can help determine if it is a contaminant or if it results from the degradation of a compound, this can be a problem. However, this is offset by the ability to detect very small amounts of material, making it suitable for measuring leachable compounds like plasticizers and other low level containments. These can then be identified using the extensive libraries for GC/MS. An example is shown in Figure 1.
Recently, PerkinElmer® has addressed this problem by the introduction of a combined TGA to FTIR to GC/MS system using its TL-9000 transfer line. In this approach, the sample is heated in the TGA to first evolve solvents and then burn the constituents to volatile compounds. The gas is transferred to the FTIR and allows for the tracking of the time or temperature dependence of a material’s evolution in the TGA. Sequentially, the gas of interest is then collected and run through the GC/MS for complete identification. This allows detections of impurities to very low levels (Figure 2). Applications include the detection of low levels of plasticizers leached from storage containers, trace levels of contaminates remaining from processing, and degradation products from exposure to higher temperature or UV radiation.
SUMMARY
Hyphenated techniques allow detection of residual solvents, leachables, and low level contaminates in pharmaceuticals, excipients, as well as other biomedical materials. Combined systems allow for the maximized performance of each to obtain the best data and to determine low levels on impurities.

Total Page Views: 7598