Issue:May 2023
IMMUNOLOGY - Innovation Gathers Momentum Amid New Scientific & Technological Breakthroughs
INTRODUCTION
The fields of immunology and immuno-oncology have seen some significant advances and changes throughout the past decade or more, all of which are bringing new hope to patients and healthcare systems. In the next 5 years, these advances are expected to gather momentum amid new scientific and technological breakthroughs.
In particular, immunology has witnessed three major trends, each crucial to continued advancement in the field. These are the next generation of immunological treatments, new approaches to research globally, and the arrival and growing importance of biosimilars. As we look forward to 2023 developments, we can expect to see some significant results in the clinic and on the market.
A NEW AGE OF IMMUNOLOGICAL TREATMENT
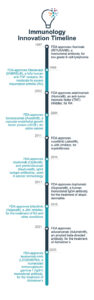
The age of immunology really began in the late 1990s and early 2000s with Tumor Necrosis Factor (TNF) alpha inhibitors and vascular endothelial growth factor (VEGF) inhibitors. Products such as infliximab (Remicade®) and adalimumab (Humira®) to treat a host of autoimmune disorders and products like bevacizumab (Avastin®) and others to treat cancer marked the arrival of this area of innovation.
The next major developments came more than a decade later with the emergence of a new generation of monoclonal antibodies, such as pembrolizumab (Keytruda®) and nivolumab (Opdivo®), to treat different types of cancer, bringing new approaches to these difficult pathologies. Since then, monoclonal antibodies have become the top selling drugs in the pharmaceutical market.1
Even more recent is the approval of several Janus kinase (JAK) inhibitors to treat a variety of chronic inflammatory disorders, such as rheumatoid arthritis, ulcerative colitis, and atopic dermatitis. These treatments tackle inflammatory conditions in a different way to classic treatments, offering hope of both symptom relief and slowing progression of disease.
Nevertheless, there have been some safety concerns raised with some JAK inhibitors, including tofacitinib (Xeljanz®) for the treatment of arthritis and ulcerative colitis, underscoring the importance of safety monitoring in late-stage clinical trials and through registries to assess their impact on a larger pool of patients.
RESEARCH REACHES THE NEXT LEVEL
New approaches to research, both with biologics and small molecule products, have led to therapies for pathologies that previously had no or very limited treatments.
Until recently, atopic dermatitis was managed with topical therapies, but research carried out by biotech and specialist pharma companies has paved the way for more holistic treatment options, including dupilumab (Dupixent®) and tralokinumab (Adbry® or Adtralza®), an injection that gained European Medicines Agency (EMA) approval in early 2021 and US Food and Drug Administration (FDA) approval later the same year.
The first treatment for active systemic lupus erythematosus in decades, anifrolumab (Saphnelo®), brings hope to patients suffering from this debilitating autoimmune disease. While used in combination with immunosuppressants, it marks a huge step forward after monoclonal and polyclonal antibody approaches proved unsuccessful.
Another disease area companies have struggled to successfully treat is Alzheimer’s disease. However, the approval of aducanumab (Aduhelm®) followed by the recent approval of lecanemab (Leqembi®) opened the door to addressing the underlying biology of the disease and slowing cognitive decline of some patients with the disease. However, concerns have been raised as to whether the benefits justify the risks, side effects, and cost of the drug.2
Perhaps one of the most prominent developments since 2020 has been in the area of mRNA research. While the best-known research and early success has been with the COVID-19 vaccines, companies are also targeting other viral diseases, such as the Zika virus and HIV, and several biotech companies have ongoing programs focused on cancer vaccines, with a number in clinical trials. There have been some promising results with an antigen specific T cell response, including prolonged disease-free survival in some clinical trials.3
One key consideration with mRNA discoveries is the complexity of scaling up good manufacturing practice (GMP)-grade production, given how new the field is from a development perspective. Equally, it will be important to set up registries or long-term follow-up of patient cohorts to monitor the safety of these products.
Beyond drug research, another area that has seen significant advances in recent years is artificial intelligence, in particular, AI and the Internet of Medical Things. These technologies are important for innovation because they provide insight into the different types of cells in the immunology system – their roles, their importance, how they interact with each other, and pathologies in the field of immune disorders – in a way that is difficult to observe through traditional human research. The observations enabled by these technologies provide new insights into potential approaches to treat or even prevent certain pathologies.
THE ADVENT OF BIOSIMILARS
While many of the innovations of the past decade or more have created new hope for patients, the cost of many of these products has been an issue for healthcare systems. Pharmaceutical companies have fought to protect their patents, but healthcare systems, particularly in Europe, have been keen to support the development of biosimilars. In September 2022, the EMA and the Heads of Medicines Agency (HMA) issued a joint statement noting that biosimilars that have been approved in the EU are interchangeable with the reference medicine or a comparable biosimilar.4
The UK’s National Health Service, for example, has welcomed biosimilars and the cost savings these create, noting after negotiations in 2018 that using the best-value adalimumab biosimilar product would save the NHS £300 million of its previously £400 million-per-year spending on the product.5
While there are development challenges to bringing a biosimilar to market, requiring expertise and investment by the companies involved, these products are broadly welcomed, at least in the EU, and make access to life-saving treatments more affordable for countries and patients. The US has been slower to adopt biosimilars, but they are starting to be seen as more attractive options by healthcare stakeholders.
INTO THE FUTURE WITH INNOVATION
The improvements in research, the arrival of new treatments on the market, and the use of novel technologies all pave the way for more breakthroughs around 2025. In 2021, for example, the FDA approved 50 innovative drugs, offering more treatment options for patients in the US.6
Importantly, many of these products are in therapeutic categories in which there is not a huge armamentarium, such as atopic dermatitis, and in areas in which there is enormous unmet need, such as Alzheimer’s disease. Research in this therapeutic category is not only being conducted with biotech products such as monoclonal antibodies, but also with small molecules. Animal studies of neuron-targeted treatment based on zinc-finger DNA-binding proteins (ZFPs) were found to lower brain levels of mutant huntingtin (mHTT) protein in a Huntington’s disease mouse model and extend survival.7
The same is true in the area of multiple sclerosis in which one biotech leader, Biogen, which has an extensive MS portfolio, has been exploring small molecule treatments for the disease in partnership with other innovator companies.8
We will also see more biosimilars come to market in the next few years, including from companies that developed the originator product as they seek to preserve their market share. As an example, Kyowa Hakko Kirin received approval in Japan for a biosimilar of its own product darbepoetin alfa for the treatment of anemia associated with chronic renal failure and cancer chemotherapy.9
PREPARING FOR 2023 WITH THE REGULATORS
As developers of innovative products look to extend their research into new areas, it is important to ensure early dialogue with the health authorities, especially during the development phase. Discussing and getting validation on the approach can help ensure development plans are in line with regulators’ expectations.
Ideally, discussions should start as soon as a company has a potential drug candidate and an idea of where they want to go with the target product profile (TPP). Given the global nature of drug development, it’s preferable to have discussions at least with the regulators in the two major regions, the US and the EU, starting with an initial meeting followed by continuous dialogue.
While there is often a belief that regulators are not supportive of drug development, the evidence is to the contrary, with most regulators eager to support innovation and help bring new products to patients in need. Whether through formal or informal discussions, regulators provide a lot of feedback and recommendations to companies that liaise with them.
The FDA, EMA, and the UK’s MHRA all have formal avenues that support development. For example, the EMA’s PRIME scheme offers enhanced interaction and early dialogue between the regulator and sponsors developing medicines that target an unmet medical need.10 FDA’s INTERACT is an informal meeting early in product development that is offered for programs that are neither too premature nor too advanced in development.11 In the UK, the Innovative Licensing and Access Pathway (ILAP) seeks to help sponsors bring their products to market faster by providing in-depth regulatory and other stakeholder input.12
Increasingly, companies are starting to appreciate the importance of initiating such a dialogue with the regulators and, as the health authorities place greater emphasis on these discussions, these interactions are likely to increase.
Other important avenues for bringing products to the market in the coming years will be to consider pathways such as compassionate use programs that allow companies to propose pre-submission use of their products for patients with serious unmet needs, such as cancer and immune disorders. Several countries in Europe have either named patient programs or in a few countries (Spain, Italy, France, and Sweden) small cohorts of patients who could possibly all be on the same protocol.
These programs not only allow patients to get the help they need sooner, but are also a tool that sponsors can use to gain greater visibility for their products by physicians, patients, and key opinion leaders, while at the same time gathering more real-world and safety data on the product. Having real-world data will become of greater importance in future as companies not only seek to demonstrate the safety and efficacy of their product, but also demonstrate its usefulness to the health technology assessment bodies for reimbursement.
One regulatory consideration for companies going into 2023 and beyond will be how to address the issue of the pediatric investigation plan (PIP). Depending on prevalence figures of disease in the pediatric population, there are likely to be tighter requirements with regard to demonstrating the product can be used by children under 16, whether the same formulation or one adapted to that population.
While this does present a constraint for companies, regulators are focused on ensuring pediatric patients, particularly those with rare or life-threatening diseases, have access to efficacious and safe treatments.
A PROMISING DEVELOPMENT FUTURE
The year ahead is likely to bring breakthroughs in drug and biologic developments. Managing the development and the regulatory pathway will require a thoughtful, preferably global strategy, focusing on patient need and the expectations of the health authorities.
REFERENCES
- Moving up with the monoclonals, Nature, Sept 2019. https://www.nature.com/articles/d43747-020-00765-2.
- Alzheimer’s disease: FDA approves lecanemab amid cost and safety concerns, BMJ, 2023. https://www.bmj.com/content/380/bmj.p73.
- mRNA vaccines — a new era in vaccinology, Nature, Jan 2018. https://www.nature.com/articles/nrd.2017.243.
- Biosimilar medicines can be interchanged, EMA, Sept 2022. https://www.ema.europa.eu/en/news/biosimilar-medicines-can-be-interchanged#:~:text=EMA_and_the_Heads_of,or_with_an_equivalent_biosimilar.
- After Biosimilar Deals, UK Spending on Adalimumab Will Drop by 75%, AJMC, Nov 2018. https://www.centerforbiosimilars.com/view/after-biosimilar-deals-uk-spending-on-adalimumab-will-drop-by-75.
- Novel Drug Approvals for 2021, FDA. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2021.
- Treatment With Zinc-finger Proteins Shows Promise in Huntington’s Mice, Huntington’s Disease News, Jan 2023. https://huntingtonsdiseasenews.com/news/use-zinc-finger-proteins-shows-promise-huntingtons-mouse-model/.
- Biogen and Skyhawk Therapeutics Announce Agreement to Develop Novel Small Molecule RNA Splicing Modifiers for Neurological Disease Targets, Biogen news release, Jan 2019. https://investors.biogen.com/ news-releases/news-release-details/biogen-and-skyhawk-therapeutics-announce-agreement-develop-novel.
- Japan approves first darbepoetin alfa biosimilar, Generics and Biosimilars Initiative, Sept 2018. https://gabionline.net/es/biosimilares/novedades/Japan-approves-first-darbepoetin-alfa-biosimilar.
- PRIME: priority medicines, EMA. https://www.ema.europa.eu/en/human-regulatory/research-development/prime-priority-medicines.
- OTAT INTERACT Meeting, FDA. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/otat-interact-meeting.
- Innovative Licensing and Access Pathway. https://www.gov.uk/guidance/innovative-licensing-and-access-pathway.

Dr. Patrick Larcier is Senior Director at PharmaLex and has worked in drug development and regulatory affairs for 30 years, at biotech companies, CROs, and in consulting. Until April 2022, he led drug development and pharmacovigilance activities for PharmaLex France and Benelux; he now provides support for PharmaLex’s growing EU and US activities in these areas.
Total Page Views: 4453