Issue:January/February 2019
FORMULATION FORUM - Sophisticated Formulation Approaches for Insoluble Drug Candidates
“Fools ignore complexity. Pragmatists suffer it. Some can avoid it. Geniuses remove it” — Alan J. Perlis
On many occasions, people have approached me with questions, such as “Are solution formulations a good, simple approach, whereas are nano-formulations too complex?” Or “What is a sophisticated formulation, and what role do nano-formulations play to accelerate the drug discovery/development process to the clinic?”
Undoubtably, there is no straight answer to those questions. As an example, for poorly water-soluble compounds with chemical instability, the development of a stable solution formulation with reasonable drug loading is a daunting task. By utilizing a nano-formulation approach, this formulation strategy may resolve the drug-loading and bioavailability issues for such compounds, while adding minimum complexity to the manufacturing process.
We all realize drug discovery and development is a very complex process requiring a stepwise and systematic approach to select compounds that have desirable therapeutic properties and are suitable for advancement into drug candidates and final drug products. A sophisticated drug delivery approach involves collaboration, technical experience, and years of working knowledge in drug development at different stages of the process. Our approach to drug development involves a thorough understanding of the physical-chemical and biopharmaceutical properties in relation to drug dissolution, absorption, and the disposition process in the body, while taking advantage of our nano-based formulation technology success. The goal being to ensure successful development of a fit-for-purpose, phase-appropriate formulation that is right the first time in an accelerated and quality manner.
There are many significant hurdles for a pharma or biotech company to overcome during the development process. The high failure rate in drug development shows that only 1 in 5,000 discovery compounds will reach the market, and 1 in every 5 drug candidates will gain approval.1 The average time from drug discovery to product launch is estimated to be ~14 years. As a result, the overall costs of drug discovery and development to bring a new medicine to the market are estimated at over $1 billion for a new chemical entity (NCE).2
Most failures in early development are mainly due to drug toxicity or safety issues, whereas a lack of efficacy is the primary reason for late-stage failure.3 The lengthy development time has been attributed to an increase in the preclinical phase to select the candidate drug. Our focus is to provide formulation and cost advantages to reduce this time in the preclinical phase. A significant increase in the percentage of NCEs with poor physical, chemical, and biopharmaceutical properties (BCS II and IV) in the drug pipeline has played a significant role in attributing to those high failure rate and increase in development timelines.4 About 50% of drugs on the market and nearly 90% of molecules in the discovery pipeline are poorly water soluble.5 Poor solubility can lead to low bioavailability, resulting in suboptimal drug delivery, ineffective drug efficacy, and side effects. As a result, various drug delivery nano-technologies, such as nano-suspensions, lipid microemulsions, nano-emulsions, and amorphous solid dispersions, can play an important role to overcome these bioavailability challenges faced by pharma and the biotech industry.
Using some of these drug development platforms, we have seen effective formulations with good bioavailability enable better assessment of the pharmacology, toxicology, safety, and efficacy properties of a compound during drug discovery and development. The results of these successful formulations can be seen in the positive pharmacokinetics, pharmacodynamics, and toxicological profiles of the drug candidate assessed in association to the biological response to specific drug targets.
Each drug discovery and development stage has its own limitations and requirements in terms of route of administration, doses, and impact of vehicle components. The objectives and specific designs of the formulation approach can vary significantly depending on the development stage. Broadly speaking, these can be classified into seven categories: 1) validation of a new target with a new pharmacology model; 2) DMPK and pharmacology for lead optimization; 3) biopharmaceutical evaluation for lead identification; 4) pre toxicological reading; 5) IND-enabling tox studies, 6) early phase (Phase 1/2a) developments; and 7) late-stage commercial development (Phase 2b & Phase 3).
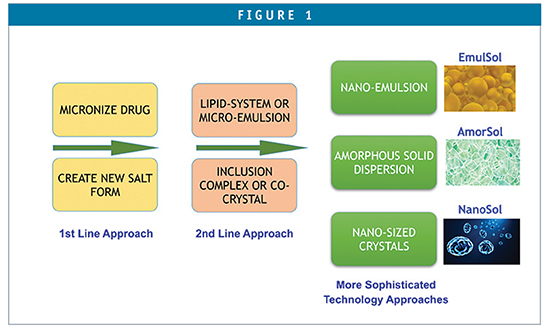
Depending on a compound’s physical, chemical, and biopharmaceutical properties, a rational formulation design can be explored with guidance from a decision tree. Numerous drugs associated with poor solubility and low bioavailability have been successfully formulated into drug products for clinical studies by a suite of available formulation technologies (Figure 1). Many marketed drugs have been successfully reformulated to improve efficacy, safety, and patient compliance using the NDA 505(b)(2) regulatory pathway. Revitalization of older marketed drug products using innovative drug delivery technologies or platforms can provide new marketing exclusivity and new patent protection, and thus offer an effective tool for product life cycle management.
Future Formulation Forums will cover a series of topics in various stages of formulation development from discovery support and candidate drug selection to development of clinical dosage forms with an emphasis on the application of chemistry, preformulation, biopharmaceutics, and novel formulation principles.
REFERENCES
- PhRMA July 2013 Profile.
- Dickson M, Gagnon JP. Key factors in the rising cost of new drug discovery and development. Nat Rev Drug Disc. 2004;3: 417-429.
- Venkatesh S, Lipper RA. Role of the development scientist in compound lead selection and optimization. J Pharm Sci. 2000;89(2):145.
- Li Di, Kerns E. Profiling drug-like properties in discovery research. Curr Opinion Chem Biol. 2003;7:402-408.
- PharmaCircle: Water Insoluble Drug Development: Strategies, Technologies, Case Studies, March 2011.
To view this issue and all back issues online, please visit www.drug-dev.com.

Jim Huang, PhD
Founder & CEO
Ascendia Pharmaceuticals
j.huang@ascendiapharma.com
www.ascendiapharma.com
Total Page Views: 5982









