Issue:March 2019
FORMULATION FORUM - Formulation Research Strategy for Discovery-Stage New Drug Candidates
“I believe things cannot make themselves impossible” – Stephen Hawking
Drug discovery medicinal chemistry programs typically involve generations of multiple lead compounds, which must be evaluated according to various selection criteria, such as:
-Selectivity of binding to target site
-Efficacy in animal disease models
-Adequate bioavailability for selected route of administration
-Adequate half-life and biodistribution
-Satisfactory exploratory toxicity in vivo
-Chemically stable and able to be synthesized at scale
The selection process allows a drug candidate along with one or more backup candidates to be chosen for preclinical development from a set of lead compounds. Thus, in vivo studies of new drug candidates at this early stage take on great importance. Negative or ambiguous results may well lead to erroneous conclusions regarding candidate advancement. In worst case scenarios, research programs may be terminated based on flawed data.
Discovery-stage formulations have intrinsic limitations (eg, limited quantities of drug candidates), and these couple with the exigencies of discovery research to introduce risks such as:
-Purity of drug substances may not be optimal
-Key physicochemical properties (eg, log P, pKa) of compounds are typically not experimentally determined
-Solid state properties (eg, crystallinity, polymorphism) are unknown
-Availability of drug substance limits number and precision of solubility measurements that can be performed
-Stability of drug substance in formulations is typically not fully evaluated
-Rapidly developed HPLC assays (eg, non-validated gradient methods) contribute some degree of inaccuracy in solubility measurements and may not give reliable indication of drug stability
Despite these limitations, formulation scientists can provide early stage formulations that manage risks, enable clear inferences from the results of in vivo studies, and allow differentiation between multiple leads. The holy grail of discovery formulation is identifying a “universal formulation” for a structurally similar lead series. This simplifies differentiation of in vivo behavior amongst several leads.
Throughout the past decade, large pharma companies have cultivated pharmaceutical scientist teams to conduct formulation researches for early stage compounds. During this same period, there has been a rapid growth of academic institutes, biotechs, and other types of start-up companies entering the drug discovery arena. However, access to discovery formulation specialists is restricted within the realm of large pharma. Even though many CROs specializing in pharmacology studies do offer formulation services, these are typically standardized concoctions that produce poorly characterized formulations and may include excipients (DMA, NMP, etc) that might not be acceptable for in vivo use in some animal models.
Therefore, it becomes critically important that CROs or CDMOs that support discovery and preclinical research have the requisite preformulation, formulation, and biopharmaceutics expertise as well as the proprietary methodologies to develop formulations from small quantities of drug candidates for different routes of administration (PO, IV, IN, IM, SC etc). An ideal approach to drug development involves a thorough understanding of the physical-chemical and biopharmaceutical properties in relationship to drug dissolution, absorption, and the disposition process in the body while taking advantage of advanced formulation technologies. A rational formulation design can be explored with guidance from a decision tree (Figure 1).
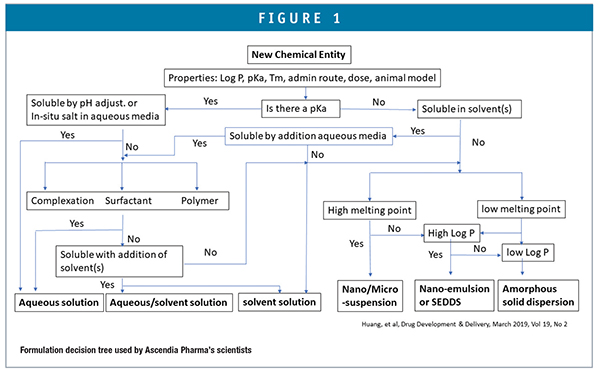
Discovery-stage formulation scientists approach drug solubilization first from consideration of the molecular structure and calculated log P and pKa values in combination with any experimental data (solubilities, melting point, etc) provided. Microscale solubility screening using co-solvents, surfactants, lipids, and complexing agents (cyclodextrins) will indicate what formulation approaches will be feasible. Particle size reduction can also be a viable approach for crystalline lead compounds. Small-volume media mills can produce nanocrystal suspensions with volumes of less than milliliters.
Often, multiple formulation approaches will be combined. Judicious choice of formulation excipients can improve solubility for a candidate by several orders of magnitude. Thus, a drug candidate may be transformed from 1 μg/ml intrinsic solubility to 100 μg/ml with concomitant improvement of the PK profile.
These early studies will often yield important experimental results that will aid subsequent development of clinically relevant dosage forms. The key outcomes from early stage formulation studies should support the emerging target product profile. Further, valuable intellectual property may develop when non-obvious biopharmceutics effects are discovered during formulation development. This can enhance the new drug entities’ patent portfolio beyond the basic composition of matter filings.
To view this issue and all back issues online, please visit www.drug-dev.com.

Jim Huang, PhD
Founder & CEO
Ascendia Pharmaceuticals
j.huang@ascendiapharma.com
www.ascendiapharma.com
Total Page Views: 6497










