Issue:September 2020
FORMULATION FORUM - Formulation Development Strategy for Early Phase Human Studies
EARLY DEVELOPMENT CHALLENGES
Successful translation of discovery compounds into first-in-human (P1) and first-in-patient (P2) is one of the key challenges facing the pharmaceutical industry. To achieve these goals, a rational formulation development strategy will be critical to avoid costly drug development failures, while speeding up the development timeline in a cost-effective manner. This is particularly true for compounds with challenging properties in solubility and bioavailability.
At the early phase of drug development, there is a limited supply of API available. Determination of an appropriate bioavailable formulation for animal PK, GLP toxicity, and first-in-human and first-in-patient is a challenging task. For poorly soluble and bioavailable compounds, development of formulations is usually achieved using drug delivery systems. Those development studies may involve the pre-formulation evaluation of the compound physicochemical biopharmaceutical properties, such as solubility and stability, in commonly used solvents and bio-relevant media, and permeability, etc. Afterward, optimization of solubility and bioavailability can be achieved by utilizing suitable delivery systems by screening a set of technology platforms, such as Ascendia’s AmorSol®, NanoSol®, and EmulSol®, that address different compound challenges in hydrophilicity, lipophilicity, and melting point.
CONSIDERATIONS FOR EARLY PHASE DEVELOPMENT
The main goal for early phase development is to test the compound safety and efficacy for the intended therapeutic indications in animal and humans. Tremendous efforts have been placed in improving the pharmacokinetic properties of compounds and ensuring bioavailability in animal models and human Phase 1 and 2 studies. Even though a simple formulation, such as solution/suspension, drug-in-capsule is always desirable to allow for a fast transition to tox and human studies, a desired compound systemic exposure in testing subjects is prerequisite to meet the goals of early development. Therefore, time and resources might have to be allocated for compounds with poor bioavailability. Otherwise, the drug program could be at a higher risk of costly failure in first-in-man and at late stages.
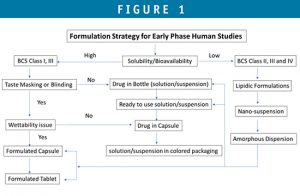
It is critical to understand the compound’s properties, route of administration, animal model, patient population, and dose range, and use a phase-appropriate formulation that can meet clinical requirements on the systemic exposure, dosing flexibility, the design of the clinical studies, and project timelines. Figure 1 is a decision tree to guide in the early formulation selection.
Below are a few points for consideration during selection of early phase formulations:
1. What is the human dose range and the correspond concentration of the selected formulation at this location?
– Rout of administration, dose requirement, and compound solubility may determine whether to choose solution or suspension.
– Dose flexibility that covers low and high dose range is important as the highest dose is unknown pending the toxicity of drug candidate in human.
– Due to the wide range of doses for early clinical phase, if solution and suspension are considered, a biopharmaceutical assessment should be conducted to assess any bridging requirement between formulations.
– Handling Instructions and stability data will be required to support each dosage form.
2. Is there a requirement in blinding for the study design?
– Taste masking may be required for certain patient populations, such as pediatrics.
– Potential taste or color difference between active and placebo.
– Use of bittering agent for liquid dosage forms.
– Use of colored packaging or dosing syringe or cups.
3. Is the clinical study conducted by in-hospital dosing or out-patient dosing?
– There is different shelf life and storage requirement for different dosing strategy.
– Ready to use solutions/suspensions with room temperature or refrigeration storage is desirable for out-patient dosing.
DOSAGE FORM OPTIONS FOR EARLY PHASE CLINICAL STUDY
Drug-in-Bottle
API compound is supplied in a bottle for constitution into solution or suspension. The reconstitution is done at a hospital pharmacy. Commercial vehicles or their modified forms, such as “Ora-Sweet,” could be considered for such purpose. The dose can be further diluted into filled bottles for different doses and for patients to take home. Dose flexibility is the advantage of the approach, and stability requirement is minimum. This dosage form is suitable for in-hospital dosing. In some cases, bulk amorphous solid dispersion formulations can be supplied in bottle and reconstituted by this approach.
Ready-to-Use Solution or Suspension
If the compound is not suspendable or dissolvable in a commonly used suspending vehicle but is stable for a longer time period of at least 3 to 6 months, a formulated solution, suspension, and nanosuspension filled in bottle at a CDMO can be considered. This dosage form is desirable for out-of-patient dosing. In some cases, lipidic formulation (SEDDS or Nanoemulsion) can be formulated and filled into bottle or hard capsules (as liquid-filled capsule) by this approach.
Drug-in-Capsule
If compound is readily wetted and dissolvable in GI fluids without the help of excipients, drug in capsule can be considered. Active compound is filled in hard capsules. Drug in capsule is suitable for out-patient dosing and for studies requiring blinding.
Formulated Capsule & Tablet
For chronic dosing or a clinical program that is planned to fast progress to late-stage phase, formulated capsule or tablet may be desired. API compound can be simply blended with a diluting excipient, and the blend is filled in hard capsules, or in some cases, for API that has challenges in flowability, wettability, and dosage uniformity, a formulated capsule or tablet may be considered. For poorly water-soluble compounds, it is possible that the enabling formulations, such as nanosuspensions, lipidic formulations, and amorphous solid dispersions, can be further incorporated into the capsule and tablet dosage forms.
SUMMARY
A phase-appropriate formulation development, which can meet clinical requirements on the systemic exposure, dosing flexibility, the design of the clinical studies, and project timelines, is important for successful translation of discovery compounds into first-in-human (P1) and first-in-patient (P2). To achieve these goals, a rational formulation development strategy is critical to avoid costly drug development failures, while also speeding up the development timeline in a cost-effective manner.
To view this issue and all back issues online, please visit www.drug-dev.com.

Jim Huang, PhD
Founder & CEO
Ascendia Pharmaceuticals
j.huang@ascendiapharma.com
www.ascendiapharma.com
Total Page Views: 9244